A new synthesis of 1,3-dihydrobenzo[1,2]oxasiloles by a novel rearrangement of a pentavalent silicon intermediate
George Bashiardes*, Vanessa Chaussebourg, Géraldine Laverdan and Jacques Pornet
Departement de Chimie, Université de Poitiers, SFA-UMR 6514, 40 avenue du Recteur Pineau, 86022 Poitiers cedex, France. E-mail: george.bashiardes@univ-poitiers.fr
First published on 26th November 2003
Abstract
A new synthesis of benzo[1,2]oxasiloles is described, wherein an unprecedented intramolecular allylic transposition takes place probably involving a pentavalent silicon intermediate.
Benzoxasiloles are interesting in their structure in that they contain the chemically labile O–Si and C–Si bonds, allowing for much derivatizing. Few methods for the synthesis of substituted benzoxasiloles have been reported, and in each case the preparations concerned mostly specific examples.1–8
We report herein a new, general synthesis of benzo[1,2]oxasiloles 1 (Fig. 1) from easily accessible ortho-silylated benzaldehydes 7a–c, for which we also describe new preparations.
![3-Allylbenzo[1,2]oxasiloles.](/image/article/2004/CC/b311804e/b311804e-f1.gif) | ||
| Fig. 1 3-Allylbenzo[1,2]oxasiloles. | ||
The reaction sequence we employed is quite general, allowing possible substitution on the allylic group and the silicon atom. Other benzaldehydes could also be employed to prepare new functionalized analogues. In Scheme 1 is described the preparation of ortho-allylsilylbenzaldehydes by two different approaches. In the first case benzaldehyde 2 is silylated with chlorosilanes 3a–c following orthometallation using N,N,N′-trimethylethylenediamine and n-butyllithium9 (method A). In the second case, ortho-bromobenzaldehyde diethylacetal 4 undergoes a halogen–metal exchange using n-butyllithium, followed by silylation to give acetals 6. Acid hydrolysis then furnishes the required ortho-substituted benzaldehydes 7 (method B). In both cases, the overall yields are good (Table 1), while the latter, two-step approach is generally higher yielding overall. In addition, a ortho-vinylsilyl benzaldehyde 7d was also prepared by the above procedures.
 | ||
| Scheme 1 Synthetic scheme for the preparation of 2-silyl benzaldehydes. i) LiNMeCH2CH2NMe2, THF, −78 °C, ii) nBuLi then iii) chlorosilane 3a–3c; iv) iPrOH, H2O, THF, TsOH. | ||
| Entry | Chlorosilane10,11 | Compound | Yield (method A) (%) | Yield (method B) (%) |
|---|---|---|---|---|
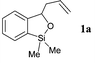
| 1 | 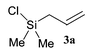 |  | 51 | 87 |
| 2 |  |  | 71 | n/a |
| 3 |  |  | other product (1c) | n/a |
| 4 | 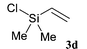 |  | 67 | 87 |
Silane derivatives 7a and 7b are then heated in toluene or xylene to effect the observed rearrangement into benzo[1,2]oxasiloles (Scheme 2). Interestingly, in one case, during the preparation of required arylallylsilane 7c (entry 3), spontaneous rearrangement under the experimental conditions (THF, 20°C), provided 1c directly (see later). As a test experiment for mechanistic elucidation, we also treated the vinylic analogue 7d in the same manner. Indeed no reaction was observed and starting material was totally recovered, which concurs with the proposed mechanism.
 | ||
| Scheme 2 | ||
Following the thermal reaction, in the cases of 7a and 7b the required 3-allyl benzo[1,2]oxasiloles 1a (R1 = H; R2 = Me) and 1b (R1 = R2 = Me) were obtained in 96% and 70% yields, respectively (Table 2). In the latter example, the remainder of the yield corresponds to recovered starting benzaldehyde 7b. 1c was obtained in 38% overall yield, directly from benzaldehyde 2, without isolation of the corresponding ortho-allylsilyl benzaldehyde 7c.
We propose a mechanism (Scheme 3) for the observed transformation by which an intermediate pentavalent silicon is formed,1,7 followed by a “nucleophilic” intramolecular allylic transposition.
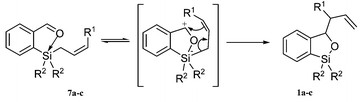 | ||
| Scheme 3 Proposed pentavalent silicon mechanism. | ||
From the observed results, it appears that the groups present on the silicon atom can influence the yield and the ease of reaction. 2b containing a Z crotyl group gave a single product albeit in lower yields certainly due to the added steric bulk of the methyl substituent on the attacking allyl moiety. It is proposed that pentavalent silicon adopts a trigonal bipyramidal configuration1,7 (Fig. 2) in which the oxygen atom occupies an axial position and that the allylic group must have an equatorial arrangement in order to transpose to the cationic benzylic methylene. A pseudo-equilibrium (rearrangement) would assure that such a relative configuration can occur. The ease of reaction observed for the triallyl analogue would suggest that such a sequence is plausible, since at all times one allyl group is at an equatorial position. Furthermore, formation of 1b as the only product confirms the concerted intramolecular character of the allylic transposition.
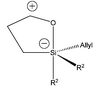 | ||
| Fig. 2 Trigonal bipyramid pentavalent silicon. | ||
No precedent for this type of allylic rearrangement has been described to our knowledge, while it has been reported2 that pentavalent silicon derivatives obtained from benzyl alcoholates do give rise to allyl, benzyl or trimethysilyl anions which can be captured by electrophiles such as benzaldehyde or benzophenone.
In order to explore the reactivity and usefulness of the compounds prepared above, benzo[1,2]oxasiloles 1a–c were transformed to 8a,b, 9a,b and 10a–c (Scheme 4). Indeed homoallylic alcohols 8a, where desilylation was achieved, were obtained by treatment with tetrabutylammonium fluoride (TBAF). Fluoride ion (KF) and hydrogen peroxide (H2O2) conditions12 provided desilylated ortho-phenolic benzyl alcohols 9a,b. Treatment of 1a–c with methyllithium13 (MeLi) resulted in nucleophilic methylation on silicon and opening of the Si–O bond to provide the silicon-containing homoallylic benzylic alcohols 10a–c.
![Transformations of benzo[1,2]oxasiloles. i) TBAF, THF, ii) KF, H2O2
(Tamao conditions), iii) MeLi, Et2O](/image/article/2004/CC/b311804e/b311804e-s4.gif) | ||
| Scheme 4 Transformations of benzo[1,2]oxasiloles. i) TBAF, THF, ii) KF, H2O2 (Tamao conditions), iii) MeLi, Et2O | ||
In all cases, the yields of the reactions were satisfactory and the compounds were obtained pure14 by flash column chromatography.
In conclusion, we describe a novel rearrangement of a pentavalent silicon intermediate generated from ortho-allylsilyl benzaldehydes to provide 1,2-benzo[1,2]oxasiloles. The method accommodates substitution on the allylic moiety and proceeds in synthetically useful to excellent yields. The benzo[1,2]oxasiloles thus obtained can be further derivatized by various methods. A general method is also described for the preparation of the ortho-silyl benzaldehydes.
Notes and references
- Y. M. Hijji, P. F. Hudrlik and A. M. Hudrlik, Chem. Commun., 1998, 1213–1214 RSC.
- P. F. Hudrlik, J. O. Arango, Y. M. Hijji, C. O. Okoro and A. M. Hudrlik, Can. J. Chem., 2000, 78, 1421–1427 CrossRef CAS.
- S. McN. Sieburth and L. Fensterbank, J. Org. Chem., 1992, 57, 5279–5281 CrossRef CAS.
- W. Ando and A. Sekiguchi, J. Organomet. Chem., 1977, 133, 219–230 CrossRef CAS.
- W. Ando, M. Ikeno and A. Sekiguchi, J. Am. Chem. Soc., 1977, 99, 6447–6449 CrossRef CAS.
- J. Belzner, H. Ihmels, L. Pauletto and M. Noltemeyer, J. Org. Chem., 1996, 61, 3315–3319 CrossRef CAS.
- Y. Yamamoto, Y. Takeda and K. Akiba, Tetrahedron Lett., 1989, 30, 725–728 CrossRef CAS.
- J. W. Fitch, III, P. E. Cassidy and M. J. Ahmed, J. Organomet. Chem., 1996, 522, 55–57 CrossRef.
- D. L. Comins and J. D. Brown, Tetrahedron Lett., 1983, 24, 5465–5468 CrossRef CAS.
- Synthesis of 3b: A. Furstner and D. Voigtländer, Synthesis, 2000, 959–969 Search PubMed.
- Synthesis of 3c: R. A. Gossage, E. Munoz-Martinez, H. Frey, A. Burgath, M. Lutz, A. L. Spek and G. van Koten, Chem. Eur. J., 1999, 5, 2191–2197 Search PubMed.
- K. Tamao, T. Kakui, M. Akita, T. Iwahara, R. Kanatani, J. Yoshida and M. Kumada, Tetrahedron, 1983, 39, 983–990 CrossRef CAS.
- R. J. P. Corriu, A. Kpoton, J. Barrau and J. Satgé, J. Organomet. Chem., 1976, 114, 21–33 CrossRef CAS.
- All compounds were verified pure by various spectral techniques including 1H and 13C NMR and gas chromatography.
| This journal is © The Royal Society of Chemistry 2004 |