Photochromism of triangle terthiophene derivatives as molecular re-router†
Tsuyoshi
Kawai
*,
Taisuke
Iseda
and
Masahiro
Irie
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, Hakozaki 6-10-1, Higashi-ku, Fukuoka 812-8581, Japan. E-mail: tkawai@cstf.kyushu-u.ac.jp
First published on 14th November 2003
Abstract
2,2′-3,3″-Terthiophene derivatives undergo photochemically reversible cyclization and cycloreversion reactions. The absorption peak wavelength changed systematically with substitution of the phenyl rings at 5-, 5′- and 5″-positions of the thiophene rings, which indicates re-routing of the π-conjugation system.
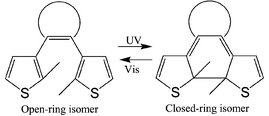
Photochromic molecules have been widely studied as photo-switching units in various photoresponsive molecules and polymers.1 Recently, photoswitching effects in photochromic diarylethenes have been extensively studied for controlling various chemical and physical properties such as refractive index, dielectric properties, electronic conduction, electrochemical response and magnetic interactions.2–9 Some of these photoswitching effects are based, at least partly, on changes in the extent of the π-conjugation in diarylethene upon the photochromic reactions. That is, the π-conjugation system of diarylethene extends over both sides of the molecule in the closed-ring form, while it is restricted on each side of its open-ring isomer, as shown in Scheme 1. The connection mode of the π-conjugation system is dependent on the substitution positions of the thiophene units to the ethene unit.10 Recently, Branda et al. have designed dithienylethene derivatives having thiophene units on their reacting carbon atoms for a photoswitching unit of the π-conjugation pathway, although various types of photocyclization reactions are possible in these molecules and the detailed reaction mechanism must be clarified.11 Since various types of molecular electronic devices have been designed on the basis of a π-conjugation connection pathway,12 photon-mode switching and re-routing of the connection mode of the π-conjugation system are worthy of extensive study.
 | ||
| Scheme 1 | ||
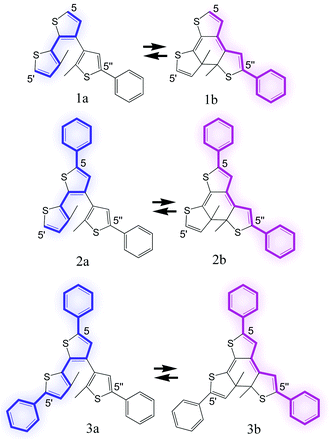
Here, we report on photochromic triangle terthiophene derivatives 1–3, which behave as photoswitching 2-way re-routers of the π-conjugation system. These molecules include a hexatriene unit in their molecular structure and are expected to undergo photoinduced cyclization and cycloreversion reactions in a similar manner to diarylethenes. As illustrated in Scheme 2, the π-conjugation system in the open-ring isomers would extend between the 5- and 5′-positions in the central and left side thiophene rings, respectively, while it would extend between the 5- and 5″-positions in the central and right side thiophene rings in the closed-ring form isomer, respectively. In order to demonstrate the re-routing effect in the π-conjugation connection mode, molecules 1–3 having different substituents were prepared.
 | ||
| Scheme 2 | ||
Compounds 1a–3a were synthesized by conventional cross-coupling reactions of thiophene derivatives and their chemical structures were confirmed by elemental analysis, mass spectra and NMR spectra.† The closed-ring isomers 1b–3b were prepared by irradiating hexane solutions of the corresponding open ring isomers 1a–3a with UV light and were isolated by HPLC from the colored solutions. Formation of the colored isomers was confirmed by mass spectrometry and/or by 1H-NMR spectroscopy in CDCl3 solution. The photochromic reaction behavior and absorption spectral changes of these molecules were studied in hexane at room temperature.
Colorless and yellow solutions of the open ring isomers 1a–3a were observed to turn red or blue depending on the molecular structures upon irradiation with UV light (λ = 313 nm). These colors disappeared upon irradiation with visible light (λ > 460 nm). These coloration and bleaching cycles can be repeated many times with alternating irradiation with UV (λ = 313 nm) and visible light (λ > 460 nm).
As shown in Fig. 1, 1a has no absorption band in the visible range and a new absorption band appeared at 558 nm upon irradiation with UV light, which corresponds to the formation of 1b. An isosbestic point was observed at 318 nm supporting the two-component photochromic reactions shown in Scheme 2. The conversion ratio from 1a to 1b was 72% at the photostationary state which was achieved by irradiation with UV light (λ = 313 nm). The colored solution was completely bleached upon irradiation with visible light. Similar photochromic performance was also observed for 2 and 3. Fig. 1(b) and (c) show absorption spectra of 1a–3a and 1b–3b, respectively. Their λmax and εmax are summarized in Table 1. The λmax of the open-ring isomers showed a systematic red shift from 1a and 3a. This is due to the introduction of phenyl groups at the 5- and 5′-positions of the thiophene rings. These spectral shifts of indicate that the π-conjugation pathway is connected between the 5- and 5′-positions and is disconnected between the 5- and 5″-positions as schematically illustrated in Scheme 2.
 | ||
| Fig. 1 (a) Absorption spectra of 1a solution (solid line) and of the photostationary state under irradiation with UV light (λ = 313 nm) (dotted line) and absorption spectrum of 1b solution (broken line). (b) Absorption spectra of open ring isomers 1a–3a. (c) Absorption spectra of closed ring isomers 1b–3b. All measurements were carried out at room temp. using hexane as solvent. | ||
| Compound | λ max/nm (ε/M−1 cm−1) | ϕ o–c b | ϕ c–o c | λ em, λex/nm |
|---|---|---|---|---|
| a All measurements were performed at room temp. in hexane solution. b At 313 nm. c At λmax of the closed ring isomers. | ||||
| 1a | 264 (20900) | 0.27 | — | 389, 313 |
| 294 (21500) | ||||
| 1b | 558 (10100) | — | 0.43 | 561, 682 |
| 2a | 272 (31500) | 0.18 | — | 432, 348 |
| 307 (28100) | ||||
| 2b | 584 (16000) | — | 0.26 | 716, 586 |
| 3a | 274 (31500) | 0.04 | — | 449, 352 |
| 308 (27000) | ||||
| 345 (26000) | ||||
| 3b | 584 (16000) | — | 0.39 | — |
On the other hand, λmax of the closed ring isomers showed a red shift in the absorption maximum upon the introduction of a phenyl ring at the 5-position of the central thiophene ring. Almost identical absorption spectra were observed for 2b and 3b. Substitution with a phenyl unit on the 5′-position does not affect the absorption spectrum. These spectral features indicate that the π-conjugation systems are connected between the 5- and the 5″-positions of the closed ring isomers but those between the 5- and 5′-positions are disconnected.
Similar spectral shifts were also observed in fluorescence emission wavelengths, as also listed in Table 1. Noted that the closed-ring isomers 1b and 2b showed fluorescence emission wavelengths different from those of 1a and 2a, respectively. That is, the emission wavelength can be modulated by the photochromic reaction, although their emission intensities were relatively weak as found for some diarylethene derivatives.13
Table 1 also summarizes the photochemical quantum yields of cyclization and cycloreversion reactions of the compounds. These values were evaluated by the standard procedure using fulgide as a standard.141 showed relatively high photochemical quantum yields in both cyclization and cycloreversion reactions. Suppressed quantum yields were observed in 3a. No marked thermal bleaching was observed in the closed ring isomers 1b, 2b and 3b at room temp.
In conclusion, the triangular terthiophene derivatives presented here showed reversible photochromic reactions with relatively high photochemical quantum yields and they can re-route the connection mode of the π-conjugation system with the photochromic reaction. We expect various kinds of photo-switching effects such as electrical conductivity and optical properties with these photo-switching units. By combining these molecular re-routers, photon-mode logic gates may be realized in the future.
The present work was partly supported by a Grant-in-Aid for the 21st Century COE Program “Functional Innovation of Molecular Informatics” and also by Grants-in-Aid for Scientific Research (#14350455 and ##15105006) from the Ministry of Education, Culture, Science, Sports and Technology of Japan.
Notes and references
-
G. H. Brown, Photochromism, Wiley-Interscience, NY, 1971 Search PubMed
; H. Durr and H. Bouas-Laurent, Photochromism: Molecules and Systems, Elsevier, Amsterdam, 1990 Search PubMed
; M. Blank, L. M. Soo, N. H. Wasserman and B. F. Erlanger, Science, 1981, 214, 70 Search PubMed
; S. Shinkai, Pure Appl. Chem., 1987, 59, 425 Search PubMed
; M. Irie, Adv. Polym. Sci., 1990, 94, 27 CAS
; M. Irie, Applied Photochromic Polymer Systems, ed. C. B. McArdle, Blackie, Glasgow, 1991, 174–206 CAS
; Chem. Rev., 2000, 100(5) CAS
: thematic issue on Photochromism: Memories and Switches; T. Ikeda and O. Tsutsumi, Science, 1995, 268, 1873 CAS
; Y. Yu, M. Nakano and T. Ikeda, Nature, 2003, 425, 145 CAS
.
- M. Irie and M. Mohri, J. Org. Chem., 1988, 53, 803 CrossRef CAS
.
- M. Irie, Chem. Rev., 2000, 100, 1685 CrossRef CAS
.
- S. Gilat, S. H. Kawai and J.-M. Lehn, J. Chem. Soc., Chem. Commun., 1993, 1439 RSC
; S.-H. Kawai, S. Gilat and J. M. Lehn, J. Chem. Soc., Chem. Commun., 1994, 1011 RSC
; S.-H. Kawai, S.-L. Gilat, R. Posinet and J.-M. Lehn, Chem. Eur. J., 1995, 1, 285 CrossRef CAS
.
- N. Tanio and M. Irie, Jpn. J.Appl. Phys., 1994, 33, 1550 CrossRef CAS
.
- T. Kawai, T. Koshido and K. Yoshino, Appl. Phys. Lett., 1995, 67, 795 CrossRef CAS
; T. Koshido, T. Kawai and K. Yoshino, J. Phys. Chem., 1995, 99, 6110 CrossRef CAS
; T. Kawai, N. Fukuda, D. Groschl, S. Kobatake and M. Irie, Jpn. J. Appl. Phys., 1999, 38, L1194 CrossRef CAS
; T. Kawai, T. Sasaki and M. Irie, Chem. Commun., 2001, 711 RSC
; J. Chauvin, T. Kawai and M. Irie, Jpn. J. Appl. Phys., 2001, 40, 2518 CrossRef CAS
.
- T. Kawai, K. Kunitake and M. Irie, Chem. Lett., 1999, 905 CrossRef CAS
.
- J. M. Endtner, F. Effenberger, A. Hartschuh and H. Port, J. Am. Chem. Soc., 2000, 122, 3037 CrossRef CAS
.
- K. Matsuda and M. Irie, J. Am. Chem. Soc., 2000, 122, 7195 CrossRef CAS
.
- K. Uchida and M. Irie, Chem. Lett., 1995, 969 CAS
; K. Uchida, T. Ishikawa, M. Takeshita and M. Irie, Tetrahedron, 1998, 54, 6627 CrossRef CAS
; K. Uchida, T. Matsuoka, S. Kobatake, T. Yamaguchi and M. Irie, Tetrahedron, 2001, 57, 4559 CrossRef CAS
; T. Fukaminato, S. Kobatake, T. Kawai and M. Irie, Proc. Jpn. Acad., 2001, 77, 30 Search PubMed
; K. Matsuda, M. Matsuo, S. Mizoguti, K. Higashiguchi and M. Irie, J. Phys. Chem., B, 2002, 106, 11218 CrossRef CAS
; C. Okabe, N. Tanaka, T. Fukaminato, T. Kawai, M. Irie, Y. Nibu, H. Shimada, A. Goldberg, S. Nakamura and H. Sekiya, Chem. Phys. Lett., 2002, 357, 113 CrossRef CAS
; T. Fukaminato, T. Kawai, S. Kobatake and M. Irie, J. Phys. Chem., B, 2003, 107, 8372 CrossRef CAS
.
- A. Peters, R. McDonald and N. R. Branda, Chem. Commun., 2002, 2275 Search PubMed
.
- R. M. Metzger, B. Chen, U. Hoepfne, M. V. Lakshmikantham, D. Vuillaume, T. Kawai, X. Wu, H. Tachibana, T. V. Hughes and H. Sakurai, J. Am. Chem. Soc., 1997, 117, 10455 CrossRef CAS
; A. Dhirani, P. H. Lin, P. Guyot-Sionnest, R. W. Zehner and L. R. Sita, J. Chem. Phys., 1997, 106, 5249 CrossRef CAS
; C. Joachim, J. K. Gimzewski and A. Aviram, Nature, 2000, 408, 541 CrossRef CAS
.
- Y. Kaneuchi, T. Kawai, M. Hamaguchi, K. Yoshino and M. Irie, Jpn. J. Appl. Phys., 1997, 36, 368 CrossRef CAS
; M.-S. Kim, T. Kawai and M. Irie, Chem. Lett., 2001, 702 CrossRef CAS
; M.-S. Kim, T. Kawai and M. Irie, Opt. Mater., 2003, 21, 271 CrossRef CAS
.
- Y. Yokoyama and Y. Kurita, Synth. Org. Chem. Jpn., 1991, 49, 364 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: analytical data for 1a, 2a and 3a. See http://www.rsc.org/suppdata/cc/b3/b311334e/ |
| This journal is © The Royal Society of Chemistry 2004 |
