Thermally reversible fluorescent polymorphs of alkoxy-cyano-substituted diphenylbutadienes: role of crystal packing in solid state fluorescence†
Riju
Davis
a,
Nigam P.
Rath
b and
Suresh
Das
*a
aPhotosciences and Photonics Division, Regional Research Laboratory (CSIR), Trivandrum-695 019, India. E-mail: sdaas@rediffmail.com; Fax: 91-471-2490186; Tel: 91-471-2515318
bDepartment of Chemistry, University of Missouri-St. Louis, St. Louis, MO 63121, USA
First published on 3rd November 2003
Abstract
Correlation of fluorescence and crystal packing in thermally interconvertible polymorphic states of octyloxy-cyano-substituted diphenylbutadiene possessing visually distinguishable fluorescence reveals that solid state fluorescence of this class of derivatives depends on their monomer–J-aggregate ratio, controlled by variations in their molecular packing.
Understanding the role of self-assembly and electronic interactions of constituent molecules in determining functional properties is a fundamental goal in material science. Polymorphism, the ability of molecules to adopt different crystal forms, is an important phenomenon,1–3 which can provide valuable information on structure–property relationships of materials. The role of molecular packing in determining solid state fluorescence and other photophysical properties of organic molecules is an area which is still not well understood4,5 and is of significant importance in the design of organic light emitting diodes.6 Here, we report on the interesting observation of thermally reversible transformation between two polymorphic states of octyloxy-cyano-substituted diphenylbutadiene (BC8) possessing visually distinguishable fluorescence. We show that the fluorescence of the polymorphic states of BC8 and related diphenylbutadienes can be explained on the basis of variations in their monomer–J-aggregate ratio, which are controlled by the molecular packing.
Alkoxy-cyano-substituted diphenylbutadienes have been reported to possess liquid crystalline phases7,8 and our initial interest in these compounds was to investigate photoinduced isothermal phase transition in these materials.8 The fluorescence spectra of these compounds in solution (λmax ∼435 nm, in toluene) are independent of the length of the alkoxy group, as expected, since the chromophore remains essentially the same. The fluorescence spectra of solid films, however, show a strong dependence on their alkoxy-chain length (Fig. 1). BC1 and BC4 exhibit green fluorescence whereas BC8 and BC12 exhibit blue fluorescence, indicating that the alkoxy-chain plays an important role in controlling their crystal packing. The fluorescence decay profiles of all the derivatives show good biexponential fits indicating the existence of two distinct emitting states.9 The fluorescence lifetimes of the butadiene derivatives measured in the solid state are much higher than those in solution. The short lifetimes in solution are due to isomerization about the double bond,8 a pathway which is unavailable in the solid state. Evidence of two species could also be obtained from their ground state absorption measured using reflectance spectroscopy (Fig. 1). Whereas the diffuse reflectance absorption spectrum of BC12 shows only a broad band with an absorption maximum centered on 385 nm, BC8 possesses an additional band in the long-wavelength region with an absorption maximum centered on 430 nm. With further decrease in the alkoxy-chain length, a relative increase in absorption in the long-wavelength region is observed with BC1 and BC4 possessing intense long-wavelength absorption bands.
 | ||
| Fig. 1 Normalized solid state fluorescence spectra; a, BC1; b, BC4; c, BC8 and d, BC12. Excitation wavelength: 360 nm and their diffuse reflectance absorption spectra. | ||
Compared to the molecular fluorescence, the red-shifted fluorescence observed in crystalline states of several aromatic hydrocarbons has been attributed to the formation of excimers.10,11 The close resemblance of the solution and solid state absorption spectra, in such systems, indicates the absorbing species to be the monomer. In the present study, the emission and absorption spectra indicate that the different emitting states arise due to excitation of distinctly different species in the ground state and not due to formation of excimers. Interaction between the chromophores in their ground state has been fairly well explained by McRae and Kasha12 in terms of exciton coupling theory, in which the excited state of aggregates splits into two energy levels (Davydov splitting). The transition to the upper state is allowed in the case of H-aggregates, characterized by a hypsochromically shifted absorption band, and that to the lower state for J-aggregates marked by a bathochromically shifted absorption band compared to the isolated monomer.12,13 The red-shifted absorption and emission observed in the solid films of these butadiene derivatives, which are absent in solution, can hence be assigned to those of the J-aggregates, whereas the short-wavelength absorption and emission bands, which correspond closely to the solution spectra, can be assigned to those of the monomer. Solid state emission arising from excited states of aggregates has been proposed for several aromatic dicarboxamide5 and azobenzene14,15 derivatives.
BC8 was observed to exist in two thermally interconvertible polymorphic forms with visually distinguishable fluorescence. Although BC8 exhibits blue fluorescence in its stable state, when obtained as its freshly solidified melt, it possesses green fluorescence. This form was found to be metastable and reverts to the stable blue fluorescent state over a period of 6 h at 27 °C (Fig. 2). The absorption and emission spectra of BC8 in its green fluorescing form match closely with those of BC1 and BC4, which are inherently green fluorescing. The corresponding changes in absorption (Fig. 3A) and emission (Fig. 3B) during this transformation were monitored at regular intervals of time.
 | ||
| Fig. 2 Solid state fluorescence of BC8 in the two fluorescing states. | ||
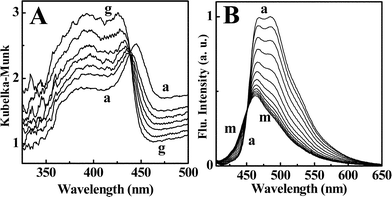 | ||
| Fig. 3 Time-dependent spectral changes in a metastable BC8 film; A) diffuse reflectance absorption spectra: (a) 0 h, (g) 6 h, in steps of 1 h; B) fluorescence spectra: (a) 0 h, (m) 6 h, in steps of 30 min. | ||
BC8 crystallizes from solvents as its blue fluorescent polymorph, and its molecular packing obtained by single crystal XRD analysis indicates an extended π-stacked structure (Fig. 4). In addition, intermolecular CH⋯O hydrogen bonds16,17 between aromatic hydrogen and oxygen atoms of adjacent molecules play an important role in defining its crystal structure.
![Crystal packing plot of BC8 projected on the a–c plane.Crystal data for BC8: C25H29NO, M
= 359.49, monoclinic, a
= 8.8931(8), b
= 6.1431(5) and c
= 37.907(3)
Å, β
= 91.066(6)°, U
= 2070.5(3)
Å3, T
= 220(2) K, space group P21/c, Z
= 4, µ
= 0.069 mm−1, 17785 reflections measured, 4072 unique (Rint
= 0.078), final R indices [I > 2σ(I)]
R1
= 0.0563, wR2
= 0.1050, R indices (all data)
R1
= 0.1320, wR2
= 0.1305. CCDC 222238. See http://www.rsc.org/suppdata/cc/b3/b311121k/ for crystallographic data in .cif or other electronic format. The dashed lines represent intermolecular CH⋯O hydrogen bonds.](/image/article/2004/CC/b311121k/b311121k-f4.gif) | ||
| Fig. 4 Crystal packing plot of BC8 projected on the a–c plane.‡ The dashed lines represent intermolecular CH⋯O hydrogen bonds. | ||
The difference in crystal packing in the two polymorphs was clearly discernable from their distinct powder XRD patterns.9 Strong diffraction peaks corresponding to an interplanar distance (d-spacing) of ∼4 Å, attributable to π-stacking,10,18 are observed in both polymorphs. The powder XRD pattern of the metastable state shows a diffraction peak corresponding to a d-spacing of 24.3 Å, which closely matches the molecular length (25.3 Å) of BC8. The largest d-spacing for the stable polymorph on the other hand is 19.7 Å. The shorter d-spacing observed in the stable state is suggestive of interdigitation of molecules, as observed in its molecular packing (Fig. 4). Based on changes in fluorescence and the powder XRD patterns we propose a mechanism schematically shown in Fig. 5 to explain the thermal transformations between the two polymorphic states of BC8.
 | ||
| Fig. 5 Schematic representation of the molecular ordering in the two polymorphic states of BC8. | ||
Melting results in disruption of the weak CH⋯O hydrogen bonds causing the molecule to move out of its interdigitated array and form separate layers of unidirectionally oriented molecular stacks as shown in Fig. 5. As a result, the π-stacks move closer to each other resulting in the formation of J-aggregates. Evidence for the J-aggregate formation in the metastable state of BC8 is also obtained from the XRD patterns of BC1 and BC4, which exhibit green fluorescence. In the XRD patterns of BC1 and BC4, peaks corresponding to their molecular lengths are observed at 15.1 and 20.8 Å, respectively.9 In BC8, the slow recovery of the stable blue fluorescing state can then be attributed to a movement of the molecules within the crystal lattice to regain the original crystal structure, the driving force for such a transformation possibly being the reformation of the CH⋯O bonds as well as dipolar–dipolar repulsion. The polymorphic behaviour was not exhibited by BC12.
In conclusion, we have described the origin of solid state fluorescence of alkoxy-cyano-substituted diphenylbutadiene derivatives as well as factors that control polymorphism in BC8. The emissions observed in these materials could be attributed to those arising from non-interacting monomers and J-aggregates. The solid state fluorescence of these materials is highly sensitive to light and heat and the use of these materials in photoimaging and thermal sensing is being explored.
RD and SD thank the Council of Scientific and Industrial Research (TF-CMM 0010) and the Department of Science and Technology, Government of India for financial assistance. This is contribution no. RRLT-PPD-163 from RRL, TVM.
Notes and references
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS.
- L. Yu, G. A. Stephenson, C. A. Mitchell, C. A. Bunnell, S. V. Snorek, J. J. Bowyer, T. B. Borchardt, J. G. Stowell and S. R. Byrn, J. Am. Chem. Soc., 2000, 122, 585 CrossRef CAS.
- R. J. Davey, N. Blagden, G. D. Potts and R. Docherty, J. Am. Chem. Soc., 1997, 119, 1767 CrossRef CAS.
- B. M. Sheikh-Ali, M. Rapta, G. B. Jameson, C. Cui and R. G. Weiss, J. Phys. Chem., 1994, 98, 10412 CrossRef CAS.
- F. D. Lewis and J.-S. Yang, J. Phys. Chem. B, 1997, 101, 1775 CrossRef CAS.
- A. Kraft, A. C. Grimsdale and A. B. Holmes, Angew. Chem., Int. Ed., 1998, 37, 403 CrossRef CAS.
- R. Brettle, D. A. Dunmur, N. J. Hindley and C. M. Marson, J. Chem. Soc., Perkin Trans. 1, 1993, 775 RSC.
- R. Davis, V. A. Mallia and S. Das, Chem. Mater., 2003, 15, 1057 CrossRef CAS.
- See supplementary information.
- B. Stevens, Spectrochim. Acta, 1962, 18, 439 CAS.
- K. Mizuno, J. Phys. Chem. Soc. Jpn., 1990, 59, 1458 Search PubMed.
- E. G. McRae and M. Kasha, in Physical Processes in Radiation Biology, L. Augenstein, R. Mason and B. Rosenberg, Eds., Academic Press, New York, 1964, pp. 23–42 Search PubMed.
- O. Valdes-Aguilera and D. C. Neckers, Acc. Chem. Res., 1989, 22, 171 CrossRef CAS.
- K. Tsuda, G. C. Dol, T. Gensch, J. Hofkens, L. Latterini, J. W. Weener, E. W. Meijer and F. C. D. Schryver, J. Am. Chem. Soc., 2000, 122, 3445 CrossRef CAS.
- J. Azuma, A. Shishido, T. Ikeda and N. Tamai, Mol. Cryst. Liq. Cryst., 1998, 314, 83 Search PubMed.
- G. R. Desiraju, Acc. Chem. Res., 1996, 29, 441 CrossRef CAS.
- S. Scheiner, S. J. Grabowski and T. Kar, J. Phys. Chem. A, 2001, 105, 10607 CrossRef CAS.
- M. Cotrait, P. Marsau, L. Kessab, S. Grelier, A. Nourmamode and A. Castellan, Aust. J. Chem., 1994, 47, 423 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: detailed spectral and fluorescence lifetime data. See http://www.rsc.org/suppdata/cc/b3/b311121k/ |
| ‡ Crystal data for BC8: C25H29NO, M = 359.49, monoclinic, a = 8.8931(8), b = 6.1431(5) and c = 37.907(3) Å, β = 91.066(6)°, U = 2070.5(3) Å3, T = 220(2) K, space group P21/c, Z = 4, µ = 0.069 mm−1, 17785 reflections measured, 4072 unique (Rint = 0.078), final R indices [I > 2σ(I)] R1 = 0.0563, wR2 = 0.1050, R indices (all data) R1 = 0.1320, wR2 = 0.1305. CCDC 222238. See http://www.rsc.org/suppdata/cc/b3/b311121k/ for crystallographic data in .cif or other electronic format. |
| This journal is © The Royal Society of Chemistry 2004 |

