Highly enantioselective spiro cyclization of 1,6-enynes catalyzed by cationic skewphos rhodium(I) complex†
Koichi
Mikami
*,
Yukinori
Yusa
,
Manabu
Hatano
,
Kazuki
Wakabayashi
and
Kohsuke
Aikawa
Department of Applied Chemistry, Tokyo Institute of Technology, Tokyo, 152-8552, Japan. E-mail: kmikami@o.cc.titech.ac.jp; Fax: +81 3 5734 2776; Tel: +81 3 5734 2142
First published on 7th November 2003
Abstract
A cationic rhodium(I) complex having a skewphos ligand is shown to be a highly enantioselective catalyst for asymmetric carbocyclization of 1,6-enynes with tri-substituted olefins to control quaternary stereogenic centers of spiro-rings.
Transition metal catalysed ene-type cyclization of 1,6-enynes1,2,3 are useful methods particularly for 5-membered rings. However, precedent examples for enantioselective catalysis with chiral metal complexes are limited to palladium4,5 and rhodium6,7 complexes, despite its synthetic potential leading not only to carbocycles but also to heterocycles.8 Recently, X. Zhang has reported excellent examples9 that a chiral rhodium complex is advantageous in terms of the facile cyclization even at room temperature but only applicable to disubstituted cis-olefin substrates. Herein, we report efficient catalysis of ene-type cyclization including tri-substituted 1,6-enynes by cationic chiral rhodium(I) complexes10 bearing a skewphos ligand.11 The chirally dynamic (tropos: turn in Greek)12 skewphos provides a deep insight into the key transition states for C–C bond formation.
First, the feasibility of the ene-type carbocyclization was investigated for a tri-substituted olefinic ether substrate 1 using 5 mol% of cationic Rh(I) catalyst including a variety of achiral bidentate PP-ligands in dichloromethane at room temperature (Table 1). Since the cationic Rh(I)(PP-ligand) dimer complexes11,13 are usually unstable to isolation or are difficult to store in solution for any length of time even at low temperature, “in-situ preparation”14 is adopted in our reactions. Interestingly, the normal ene-type cyclization proceeds even with such a sterically congested tri-substituted olefinic substrate, but along with the unexpectedly isomerised endo-cyclic secondary products 3 with dppe (1,2-bis(diphenylphosphino)ethane) (entry 1). By contrast, dppp (1,3-bis(diphenylphosphino)propane) and dppb (1,4-bis(diphenylphosphino)butane) gave the desired ene-type cyclization products 2 with high regioselectivities (entries 2 and 3).
Next we examined the chiral PP-ligands such as (S)-BINAP and (S,S)-skewphos ((2S,4S)-2,4-bis(diphenylphosphino)pentane, (S,S)-BDPP)13 corresponding to dppp and dppb skeletons, respectively. The key to the success in increasing the enantioselectivities and olefinic regioselectivities in carbocyclization is the use of skewphos rather than BINAP (entry 4) to give 93% ee and 90% olefinic regioselectivities at room temperature (entry 5). Even at 0 °C, reaction with (S,S)-skewphos proceeded slowly, and moreover olefinic regioselectivity increased up to 98% with maintaining chemical yield and enantioselectivity of the major product 2 (entry 6).
Furthermore, catalytic asymmetric cyclization of amide substrate 4 using Rh(I)/(S,S)-skewphos complex proceeded in moderate yield but without olefin migration, to afford 5 involving quaternary carbon center as a sole product (Eqn. 1).
 | (1) |
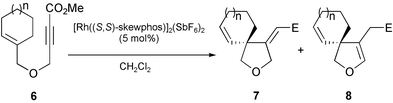
Encouraged by these interesting results among tri-substituted substrates, we next tried the spiro-cyclizations of ether compounds 6 catalyzed by chiral cationic Rh(I)/(S,S)-skewphos complex (Table 2). Cyclization of 6a with 6-membered ring was executed at room temperature for 46 hours to give 7a in 53% yield and 88% ee, together with olefin-migration product 8a with high enantiomeric excess (97% ee).15
The reactivity increased dramatically at 80 °C and the cyclization of 6a completed within only 40 minutes but 8a was obtained in high enantioselectivity (44%, 91% ee) (entry 2). For 6b involving 8-membered medium ring, the major product was 8b under either 40 °C or 80 °C conditions, although the enantioselectivity was high (88% ee and 79%ee, respectively) (entry 3 and 4).
To increase the synthetic usefulness, spiro-cyclization16 of pyran amide compound 9 was executed (Eqn. 2). Spiro cyclization proceeds smoothly at room temperature to afford the desired spiro amide-pyran 10 with extremely high enantioselectivity of 94% ee without accompanying any olefin migration.
 | (2) |
It should be noted that the transition states for C–C bond formation9c indicate the chirally dynamic (tropos) nature of the skewphos ligand. Skewphos is known to have both chair and skew forms and these forms exist in equilibrium with each other17 (Fig. 1). As its name shows, skewphos is stable in the favourable skew conformation (λ- over δ-skew forms).16,17 In fact, due to their uncontrollable nature, examples of asymmetric catalysis using skewphos analogues are very limited although some chiral skewphos analogues have been devised.18–20 Undoubtedly, BINAP has a rigid (atropos: not turn in Greek)12 binaphthyl skeleton and cannot easily epimerize. (S)-Enriched cyclized product 2 was obtained by enantiopure and atropos (S)-BINAP,20,21 which has (I,III)-equatorial phenyl groups in the Rh-quadrant (Table 1, entry 4). On the other hand, we obtained (R)-enriched cyclized product 2 by (S,S)-skewphos, in sharp contrast to the (R)-product obtained with (S)-BINAP (Table 1, entries 5 and 6). In our ene-type cyclization, (S,S)-skewphos should possess the less stable δ-skew form in (I,III)-equatorial conformation as does (S)-BINAP. Therefore it is indicated that our cationic Rh(I)-catalyzed ene-type cyclizations should proceed via the less favourable λ-skew form. In the λ-skew form, the (II,IV)-equatorial phenyl groups are inclined a little from the horizontal because of the repulsion between two axial-Me groups of skewphos and these Ph groups. In quadrants II and IV in the δ-skew form, a vacant space is available for the bulky alkyl group of the substrate, leading to (R)-enriched product 2 without conspicuous repulsions.
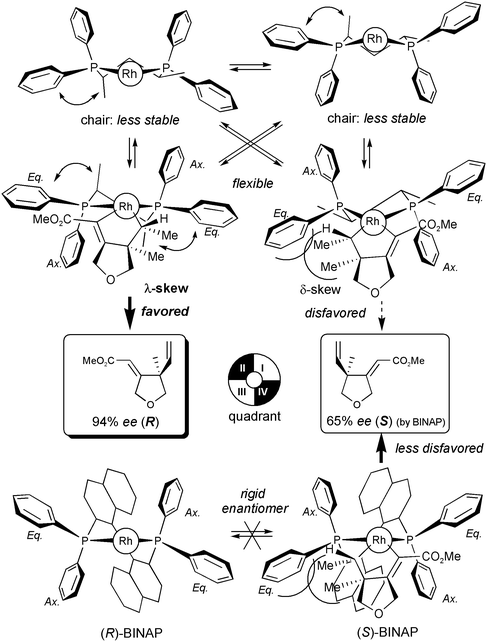 | ||
| Fig. 1 Flexible skew-conformation of skewphos vs. rigid BINAP. | ||
In summary, we have developed highly enantioselective spiro cyclization of 1,6-enynes with tri-substituted olefin catalysed by cationic skewphos rhodium(I) complex. This is the first example of spiro-constructions by Rh(I) catalysis via ene-type cyclization. Further mechanistic analyses and crystallization of cationic Rh(I) complexes for X-ray analyses are now under way.
Notes and references
- For reviews: (a) B. M. Trost and M. J. Krische, Synlett, 1998, 1–16; (b) B. M. Trost, Acc. Chem. Res., 2002, 35, 695–705 CrossRef CAS.
- (a) E. Negishi, C. Copéret, S. Ma, S. Liou and F. Liu, Chem. Rev., 1996, 96, 365–393 CrossRef CAS; (b) C. Aubert, O. Buisine and M. Malacria, Chem. Rev., 2002, 102, 813–834 CrossRef CAS.
- For an excellent textbook: J. Tsuji, Palladium Reagents and Catalysts, John Wiley & Sons, Chichester, 1995 Search PubMed; J. Tsuji, Transition Metal Reagents and Catalysts: Innovations in Organic Synthesis, John Wiley & Sons, Chichester, 1995 Search PubMed.
- (a) B. M. Trost, D. C. Lee and F. Rise, Tetrahedron Lett., 1989, 30, 651–654 CrossRef CAS; (b) B. M. Trost and B. A. Czeskis, Tetrahedron Lett., 1994, 35, 211–214 CrossRef CAS; (c) A. Goeke, M. Sawamura, R. Kuwano and Y. Ito, Angew. Chem. Int. Ed. Engl., 1996, 35, 662–663 CrossRef CAS.
- (a) M. Hatano, M. Terada and K. Mikami, Angew. Chem. Int. Ed., 2001, 40, 249–253 CrossRef CAS; (b) M. Hatano and K. Mikami, J. Mol. Cat. A: Chemical, 2003, 196, 165–169 CrossRef CAS; (c) M. Hatano and K. Mikami, J. Am. Chem. Soc., 2003, 125, 4704–4705 CrossRef CAS.
- P. Cao and X. Zhang, Angew. Chem. Int. Ed., 2000, 39, 4104–4106 CrossRef CAS.
- Achiral Rh(I)-catalyzed cycloisomerization of allenenes: T. Makino and K. Itoh, Tetrahedron Lett., 2003, 44, 6335–6338 Search PubMed.
- L. S. Hegedus, Transition Metals in the Synthesis of Complex Organic Molecules, University Science Books, Mill Valley, CA, 1999 Search PubMed.
- (a) A. Lei, M. He and X. Zhang, J. Am. Chem. Soc., 2002, 124, 8198–8199 CrossRef CAS; (b) A. Lei, M. He, S. Wu and X. Zhang, Angew. Chem. Int. Ed., 2002, 41, 3457–3460 CrossRef; (c) A. Lei, J. P. Waldkirch, M. He and X. Zhang, Angew. Chem. Int. Ed., 2002, 41, 4526–4529 CrossRef CAS.
- A. Miyashita, H. Takaya, T. Souchi and R. Noyori, Tetrahedron, 1984, 40, 1245–1253 CrossRef CAS.
- J. Halpern, D. P. Riley, A. S. C. Chan and J. J. Pluth, J. Am. Chem. Soc., 1977, 99, 8055–8057 CrossRef CAS.
- For review: K. Mikami, K. Aikawa, Y. Yusa and M. Yamanaka, Synlett, 2002, 1561–1578 Search PubMed.
- In earlier publication, the abbreviation BDPP is used. J. Bakos, I. Tóth, B. Heil and L. Markó, J. Orgamomet. Chem., 1985, 279, 23–29 Search PubMed.
- For the preparation of the cationic Rh(I)-dimer complexes from [Rh(nbd)Cl]2 precursor, see ESI..
- Double bond migration of cyclic olefin, that occurs in Pd-catalysis, were not observed in Rh-catalysis. See ref. 4b: Also see (a) F. Ozawa, A. Kubo, Y. Matsumoto and T. Hayashi, Organometallics, 1993, 12, 4188–4196 CrossRef CAS; (b) O. Loiseleur, P. Meier and A. Pfaltz, Angew. Chem. Ind. Ed. Engl., 1996, 35, 200–201 Search PubMed.
- A double ring closing metathesis by Ru: D. J. Wallace, J. M. Goodman, D. J. Kennedy, A. J. Davies, C. J. Cowden, M. S. Ashwood, I. F. Cottrell, U.-H. Dolling and P. J. Reider, Org. Lett., 2001, 3, 671–674 Search PubMed.
- J. Bakos, I. Tóth, B. Heil, G. Szalontai, L. Párkányi and V. Fülöp, J. Organomet. Chem., 1989, 370, 263–276 CrossRef CAS.
- Rh-catalyzed asymmetric hydrogenation: P. A. MacNeil, N. K. Roberts and B. Bosnich, J. Am. Chem. Soc., 1981, 103, 2273–2280 Search PubMed.
- Ru-catalyzed asymmetric hydrogenation: C. Bianchini, P. Barbaro, G. Scapacci and F. Zanobini, Organometallics, 2000, 19, 2450–2461 Search PubMed.
- Pt-catalyzed asymmetric hydroformylation of styrene: (a) I. Tóth, I. Guo and B. E. Hanson, Organometallics, 1993, 12, 848–852 CrossRef CAS; (b) I. Tóth, C. J. Elsevier, J. G. Vries, J. Bakos, W. J. J. Smeets and A. L. Spek, J. Organomet. Chem., 1997, 540, 15–25 CrossRef CAS; (c) C. Hegedüs, J. Madarász, H. Gulyás, A. Szoöllösy and J. Bakos, Tetrahedron: Asymmetry, 2001, 12, 2867–2873 CrossRef CAS.
- The other transition state with (S)-BINAP leading to (R)-2 gives considerably more repulsion between axial-Ph in quadrant IV and the alkyl groups of the substrate..
Footnote |
| † Electronic supplementary information (ESI) available: typical experimental procedure and spectral data for 1–10. See http://www.rsc.org/suppdata/cc/b3/b310789b/ |
| This journal is © The Royal Society of Chemistry 2004 |