Carbon nanotube screen-printed electrochemical sensors
Joseph
Wang
* and
Mustafa
Musameh
Department of Chemistry and Biochemistry, New Mexico State University, Las Cruces, NM 88003, USA
First published on 9th December 2003
Abstract
The fabrication, and evaluation of carbon-nanotube (CNT)-derived screen-printed (SP) electrochemical sensors based on a CNT ink are reported. The fabricated CNT strips combine the attractive advantages of CNT materials and disposable screen-printed electrodes. Such thick-film CNT sensors have a well-defined appearance, are mechanically stable, and exhibit high electrochemical reactivity.
The unique electronic, chemical, and mechanical properties of carbon nanotubes (CNT) make them extremely attractive for electrochemical sensors.1 Recent studies demonstrated the ability of CNT to promote electron transfer reactions of important compounds and to impart higher stability onto electrochemical devices.2–7 The attractive low potential detection of hydrogen peroxide7 and NADH3 at CNT-based transducers suggests great promise for dehydrogenase- and oxidase-based amperometric biosensors. Such sensing opportunities of CNT materials have been documented in connection to CNT-coated electrodes,2,4,7 or using CNT/binder composite electrodes.8,9
The goal of this work was to develop a production method for CNT-based screen-printed electrodes. The screen printing technology is a well established technology for the mass production of disposable electrochemical sensors.10,11 While various conducting inks are viable for printing the working electrodes, carbon-based inks are commonly used owing to their low cost, wide potential window, and low background currents. Such inks are composed of graphite particles, a polymeric binder and other additives. By replacing the graphite with CNT we obtained thick-film sensor strips with improved electrochemical properties and analytical performance. The newly developed inks thus combine the attractive advantages of CNT materials and screen-printed electrodes. To our knowledge, CNT-based inks have not been used for electroanalytical work, although thick-film CNT devices have been described in connection to emission display applications.12,13
Amperometric experiments were performed with a Bioanalytical Systems (BAS) CV-27 voltammograph, in connection to a BAS X–Y recorder. Cyclic voltammograms were recorded with the Autolab PGSTAT10 Electrochemical Analyzer (Eco Chemie BV, Netherlands). The working electrode, the Ag/AgCl reference electrode (CH Instruments, Austin, TX), and platinum wire counter electrode were inserted into the 20 ml cell through holes in its Teflon cover. Scanning electron microscopic (SEM) images were obtained using the Hitachi S-3200N unit. Optical microscopic images were obtained using a Nikon A4-B light microscope connected to a Sony CCD/RGB color video camera.
All solutions were prepared from double-distilled water. Glucose oxidase (GOx, EC 1.1.3.4, Type X–S from Aspergilus niger, 157,500 U g−1 solid), β-D(+) glucose, NADH (β-nicotinamide adenine dinucleotide, reduced form), potassium dihydrogen phosphate, and dipotassium hydrogen phosphate, were purchased from Sigma. Hydrogen peroxide (30 wt.%), potassium ferricyanide, catechol, Nafion solution (5 wt.% in a mixture of lower aliphatic alcohols), isophoron, polyvinyl chloride (PVC), DBE-4 (dibasic ester containing 98.4% dimethyl succinate and 0.3% dimethyl glutarate), and DBE-5 (dibasic ester containing 99% dimethyl glutarate and 0.4% dimethyl succinate) were purchased from Aldrich (Milwaukee, WI). The graphite powder (grade #38) was received from Fisher Scientific (Fair-Lawn, NJ). Multi-wall carbon nanotubes (MWCNT), with a ∼95% purity, were obtained from NanoLab Inc. (Brighton, MA); further purification was accomplished by stirring the CNT in 2 M nitric acid for 24 h. The Acheson carbon ink (Electrodag 440B) was obtained from Acheson Colloids Co. (Ontario, CA), while the insulator was obtained from Electro-Science Laboratories (Type 240-SB, King of Prussia, PA). Alumina ceramic plates were received from CoorTek Inc. (Golden,CO).
The multi-wall CNT screen-printed electrodes were fabricated using a MWCNT-derived ink prepared by the following procedure. Purified MWCNT were ground with pestle and mortar for 10 min until a very fine powder was obtained. Then, 60 mg of that powder were mixed thoroughly with 500 µl of isophorone solution containing 2% (w/v) PVC, 2% (v/v) DBE-4, and 2% (v/v) DBE-5 until a homogeneous ink was achieved. The home-made graphite ink was prepared in a similar fashion, and the solvent was allowed to evaporate for 1 h in the fume hood. Another carbon SP electrode was prepared directly using the commercial Acheson ink. The different layers were printed on alumina ceramic plates by hand printing using the corresponding inks, patterned stencil screens, and a rubber squeezer. The printed CNT electrodes were subsequently cured for 1 h (at 150 °C) and were allowed to cool to room temperature. An insulator layer was then printed to cover most of the printed carbon strip, leaving a 2.0 mm × 5.0 mm working area, and a contact area (on the opposite side). Glucose oxidase was immobilized onto the strip electrode by casting 10 µl solution (0.05 M phosphate buffer, pH 7.4) containing 0.2 mg ml−1 GOx and 0.5% Nafion and allowing it to evaporate at room temperature for 1 h. Subsequently, the surface was rinsed with double-distilled water and stored at 4 °C.
Most measurements were carried out in a phosphate buffer (0.05 M, pH 7.4) supporting electrolyte medium; a 0.1M KCl solution was used for measurements of potassium ferricyanide. Amperometric detection proceeded under forced-convection batch conditions. All measurements were performed at room temperature.
Electrodes fabricated from CNT-derived inks are mechanically strong (with good resistance to mechanical abrasion) and possess an excellent adhesion to the ceramic substrate. Their well-defined appearance is similar to that of conventional screen-printed electrodes. Fig. 1(A and B) shows optical images for the Acheson and CNT SP surfaces. Such microscopic visualization reveals distinct smoothed (undistorted) conductor edges with minimal defects. Apparently, the printing quality is not compromised by the use of a CNT-derived ink. The printed CNT surface remained rigid and stable after several washings and electrochemical measurements. The resulting CNT strips have a resistance of 2400 ohm, compared to 236000 ohm and 220 ohm for strips based on the graphite-based and Acheson inks, respectively.
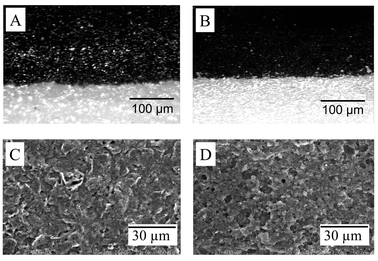 | ||
| Fig. 1 Optical microscopic images of the surfaces of the Acheson-based (A) and the CNT (B) SP electrodes. SEM images for the surface of the Acheson-based (C) and MWCNT (D) SP electrodes. Accelerating voltage, 14 kV. | ||
Fig. 1 also displays typical SEM images of the SP strips prepared from conventional-carbon (C) and CNT-derived (D) inks. The surface of the CNT-based electrode is characterized by a microporous structure of flake-shaped particles with nonuniform distribution, along with large cavities. A similar appearance, with a different flake shape and a lower porosity is indicated for the conventional SP electrodes. The different porosities (and actual surface areas) are reflected by the values of the differential capacitance (30 vs. 0.138 µF mm−2 for the CNT and Acheson based strips, respectively), and the corresponding background current. Note also that since CNT is a highly porous material, electrolytes are likely to access the interior surface leading to high differential capacitance.
Fig. 2 compares cyclic voltammograms for hydrogen peroxide (A), NADH (B), potassium ferricyanide (C), and catechol (D) at the carbon and CNT-derived SP electrodes (dashed and solid lines, respectively). For all four redox couples, the thick-film CNT sensor exhibits a higher electrochemical activity; particularly pronounced are the improvements observed for hydrogen peroxide and NADH that display a poorly defined response at the conventional electrode. Such enhancement has a major implication on the design of CNT-based enzyme sensor strips. The NADH and hydrogen peroxide oxidation starts at around 0.30 and 0.50 V, respectively, compared to 0.65 V and 0.80 V at the conventional carbon strip. A greatly improved electrochemical reversibility is also observed for potassium ferricyanide and catechol with 400 mV and 250 mV lowering of the anodic peak potentials, respectively.
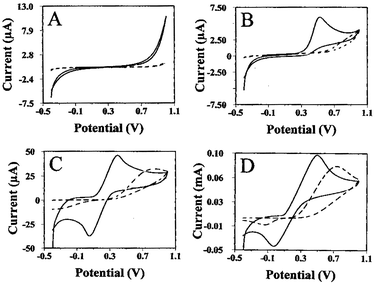 | ||
| Fig. 2 Cyclic voltammograms at the carbon/Acheson-(dashed line) and CNT-(solid line) based SP electrodes for 5 mM of hydrogen peroxide (A), NADH (B), potassium ferricyanide (C), and catechol (D). Electrolyte, phosphate buffer (0.05 M, pH 7.4) (A,B,D) and 0.1M potassium chloride (C); scan rate, 50 mV s−1. | ||
The electrocatalytic action of the CNT sensor strip facilitates low potential amperometric measurements of hydrogen peroxide and NADH. Fig. 3 displays the amperometric response of the conventional (a) and CNT (b) strip electrodes to successive additions of 2 mM hydrogen peroxide (A) and 0.1 mM NADH (B). As expected from the cyclic voltammetric data (of Fig. 2), the conventional carbon strip is not responsive to these analytes at the low operating potentials of +0.3 (A) and +0.2 (B) V. In contrast, a well defined and fast (∼10 s) response is observed at the CNT SP electrode. The favorable signals are accompanied by a low noise level. Also shown are the resulting (non-linear) calibration plots.
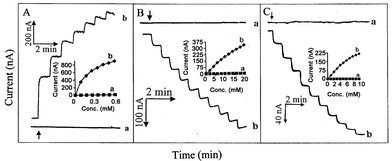 | ||
| Fig. 3 Current–time recordings obtained at the carbon/Acheson (a) and CNT (b) SP electrodes upon increasing the concentration of hydrogen peroxide (A) and NADH (B) in steps of 2 mM and 0.1 mM, respectively, and for successive 1 mM additions of glucose (C) at the carbon/GOx (a) and CNT/GOx (b) SP electrodes. Operating potential, +0.3 (A), +0.2 (B), and +0.10 (C) V; supporting electrolyte, phosphate buffer (0.05 M, pH 7.4); stirring rate, ∼400 rpm. Inset shows the resulting calibration plots for hydrogen peroxide (A), NADH (B), and glucose (C). | ||
The effective electrocatalytic detection of hydrogen peroxide indicates great promise for amperometric biosensing of glucose (and other substrates of oxidase enzymes). Fig. 3C compares the amperometric response to successive additions of 1 mM glucose at the carbon/GOx (a) and CNT/GOx (b) SP electrodes using a low operating potential of +0.1 V, along with the resulting calibration plots (insets). As expected, only the CNT-based thick-film biosensor responds favorably to the glucose additions at this potential. Such low-potential electrocatalytic detection commonly minimizes contributions from co-existing electroactive constituents and hence offers high selectivity. For example, the response for 4 mM glucose was not affected by additions of 0.1 mM acetaminophen and uric acid; in contrast, some interference was observed in the presence of 0.1 mM ascorbic acid (detection potential, 0.0 V). The latter reflects the electrocatalytic oxidation of ascorbic acid at CNT-modified surfaces,6 and would require the use of a permselective coating. The performance displayed in Fig. 3C, particularly the low operating potential, compares favorably with that of mediator-based glucose strips.
This work was supported by grants from the US EPA (Award Number RD830900). MM acknowledges a fellowship from IDB (Jeddah, SA).
References
- Q. Zhao, Z. Gan and Q. Zhuang, Electroanalysis, 2002, 14, 1609a CrossRef CAS.
- J. Wang, M. Li, Z. Shi, N. Li and Z. Gu, Electroanalysis, 2002, 14, 225 CrossRef CAS.
- M. Musameh, J. Wang, A. Merkoci and Y. Lin, Electochem. Commun., 2002, 4, 743 Search PubMed.
- J. Wang, M. Li, Z. Shi, N. Li and Z. Gu, Anal. Chem., 2002, 74, 1993 CrossRef CAS.
- Y. Zhao, W. D. Zheng, H. Chen and Q. M. Luo, Talanta, 2002, 58, 529 CrossRef CAS.
- Z. H. Wang, J. Liu, Q. L. Liang, Y. M. Wang and G. Luo, Analyst, 2002, 127, 653 RSC.
- J. Wang, M. Musameh and Y. Lin, J. Am. Chem. Soc., 2003, 125, 2408 CrossRef CAS.
- J. Wang and M. Musameh, Anal. Chem., 2003, 75, 2075 CrossRef CAS.
- M. D. Rubianes and G. A. Rivas, Electrochem. Comm., 2003, 5, 689 CrossRef CAS.
- S. Wring and J. Hart, Analyst, 1992, 117, 1281 RSC.
- J. Wang, B. Tian, V. Nascimento and L. Angnes, Electrochim. Acta, 1998, 43, 3459 CrossRef CAS.
- D. Lee, M. Yi, H. Jung, W. Seo, J. Park, H. Chun and N. Koh, Thermec' 2003, 2003, 426–4, 2297 Search PubMed.
- Y. Li, C. Zhu and X. Liu, Diamond Relat. Mater., 2002, 11, 1845 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
