Immunoquantitative analysis for berberine and its related compounds using monoclonal antibodies in herbal medicines
Jun-Sik
Kim
,
Hiroyuki
Tanaka
and
Yukihiro
Shoyama
*
Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan
First published on 11th December 2003
Abstract
The monoclonal antibody (MAb) against berberine, a bioactive constituent of Coptis japonica M., Phellodendron amurense R. and Hydrastis canadensis L., was produced and characterized. As immunogen, the derivative of berberine, 9-O-carboxymethyl berberrubine was synthesized and conjugated to carrier protein, bovine serum albumin (BSA). In order to confirm its immunogenicity, the ratio of hapten in berberine–BSA conjugate was determined by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS). After immunization, hybridomas secreting MAbs against berberine were produced by fusing splenocytes with mouse myeloma cell line, P3-X63-Ag8-653. After the screening, anti-berberine MAb 1D5-3B-7 was obtained. Subsequently, a quantitative ELISA system for berberine and its related compounds using the MAb was established and evaluated comparing with HPLC method. The ELISA method described in this study can be available as an analytical tool for quality control and standardization of medicinal plants and its prescriptions containing berberine and its related compounds.
Introduction
Berberine, a quarternary protoberberine-type alkaloid, is one of the well-known bioactive compounds distributed widely in a number of clinically important medicinal plants such as Coptis japonica M., Phellodendron amurense R. and Hydrastis canadensis L. Berberine containing plants have been used in traditional and folk medicine around the world for centuries. It has been used for a bitter tonic, diaphoretic, anti-pyretic, eye infections and diarrhoea.1 In the past three decades, berberine has been studied intensively in over 1000 cases because of its physiological and pharmacological effects such as anti-inflammatory,2 anti-fungicidal,3 anti-secretory,4 anti-mycotic,5 anti-leishmanial6 and cardiovascular effect.7 Therefore, berberine has been applied orally as a tea or capsule, externally as a skin lotion, as an eyewash, or as a virginal douche. In addition to its medicinal uses, berberine is also used as a fluorescent staining of cells,8 DNA,9 and energized mitochondria10 in biochemical researches. Currently, a number of methods including colorimetric determinations,11 thin layer chromatography (TLC),12 high performance liquid chromatography (HPLC)13,14 and capillary electrophoresis (CE)15 have been applied for the determination of berberine in its quality control. Although these chromatographic methodologies are accurate and sufficiently sensitive, there are some disadvantages such as the limited number of samples, pretreatment of sample, time and labour consuming besides interference by other compounds included in herbal medicines. In contrast, an enzyme-linked immunosorbent assay (ELISA) using monoclonal antibody (MAb) is more fast, convenient, and economic comparing with other methods. Despite their high cost and the long process involved in their development and establishment, an ELISA has become an important methodology for the quantitative and/or qualitative analysis of pesticides,16 preservatives,17 environmental chemicals18 and phytochemical compounds19 having small molecular weights.We report herein an ELISA system using MAb against berberine and its related compounds. It is applied to the analysis of berberine and its related compounds in individual plants and herbal medicines.
Experimental
Chemicals and immunochemicals
Berberine hydrochloride was purchased from Nacalai tesque, Inc. (Kyoto, Japan). Coptisine chloride, palmatine chloride, corydaline and papaverine hydrochloride were purchased from Wako Pure Chemical Ind., Ltd. (Osaka, Japan). Berberrubine, 9-acetylberberine and 8-oxyberberine were synthesized (data not shown). Prescription samples were purchased from Teikoku Seiyaku Co., Ltd. (Kagawa, Japan) and Tsumura Co. (Tokyo, Japan). Bovine serum albumin (BSA) and human serum albumin (HSA) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Enriched RPMI1640-Dulbecco's-Ham's F12 medium (eRDF) and RD-1 additives were obtained from Kyokuto Pharamceutical Ind., Ltd. (Tokyo, Japan). Peroxidase-labelled anti-mouse IgG was provided by Organon Teknika Cappel Products (West Chester, PA, USA). Fetal Calf Serum (FCS) was provided by Cambrex Co. (Wakersville, MA, USA). All other chemicals were standard commercial products of analytical grade.Synthesis of hapten for conjugates
Berberine was derived to 9-O-carboxymethyl berberine as follows (Fig. 1):Step A: Demethylation of berberine
Demethylation of berberine was performed following Iwasa's method20 with modification as indicated below. Berberine (3 g) was heated at 190 °C for 15 min. The resulting mixture was dissolved in H2O, and extracted with chloroform 3 times. Chloroform was evaporated, and the residue was purified by column chromatography using Sephadex LH20 and ODS gel to give berberrubine which was identified by the comparison of 1H NMR with that of an authentic sample.
Step B: Carboxymethylation of berberrubine
9-O-Carboxymethyl berberrubine was synthesized following Sadykov's method with modification. Chloroacetic acid (300 mg, 3.17 mol) was added to a stirred solution of berberrubine (100 mg, 0.31 mol) in chloroform at room temperature.21 The reaction mixture was stirred for 3 h. The solvent was evaporated, the residue was chromatographed to give 9-O-carboxymethyl berberrubine. 9-O-Carboxymethyl berberrubine was identified by comparing the data of 1H NMR with that of an authentic sample.
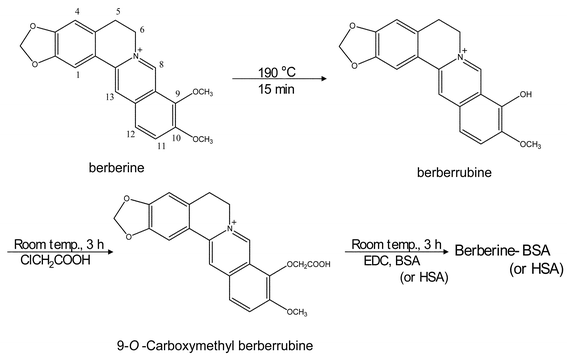 | ||
| Fig. 1 Synthesis of the hapten for conjugation with carrier protein 9-O-Carboxymethyl berberrubine preserved the chemical structure of berberine was designed for hapten and synthesized through the demethylation and carboxymethylation step following Iwasa's method and Sadykov's method. | ||
Conjugation to carrier proteins
1-Ethyl-3-(3′-dimethylaminopropyl)-carbodiimide hydrochloride (EDC, 8 mg, 0.042 mmol) and 9-O-carboxymethyl berberrubine (8 mg, 0.021 mmol) was added to a solution of pyridine (1 ml). The reaction mixture was added dropwise to H2O solution (1 ml) containing BSA or HSA (8 mg), and then stirred for 3 h at room temperature. Subsequently, the mixture was dialyzed with 5 changes of H2O for 2 days at 4 °C, and lyophilized.Determination of hapten number in hapten–carrier protein conjugate by MALDI-TOF MS
The hapten number in the berberine–protein conjugates were determined by MALDI-TOF MS as previously described.22 A small amount (1–10 pmol) of hapten–conjugate was mixed with a 103-fold molar excess of sinapinic acid in an aqueous solution containing 0.15% trifluoroacetic acid. The mixture (0.5–1.0 µl) was deposited on sample plate and air-dried. The mass spectra were acquired using a delayed extraction MALDI TOF MS (Voyager Elite, PerSeptive Biosystems, Inc., Framingham, MA, USA) operated in the linear model. The instrument was generally operated at an accelerating voltage of 20 kV and a grid voltage of 90% of the accelerating voltage. Flight time was measured by a 500 MHz transient digitizer board in the computer, and the data were analyzed using GRAMS/386 software (Galactis Industries Corp., Salem, NH, USA). The molecular weight of each conjugate was calculated from the peak centroid of the [M + H]+ peak.Immunization, hybridization and antibody production
Two female, 6 weeks old BALB/c mice (Kyudo, Kumamoto, Japan) were immunized intraperitoneally with berberine–BSA solution three times biweekly. The first immunization (50 µg of immunogen) was injected as a 1:1 (v/v) emulsion in Freund's complete adjuvant (DIFCO Laboratories., MI, USA) to a total volume of 0.5 ml. The second immunization (50 µg of immunogen) was injected as a 1:1 emulsion in Freund's incomplete adjuvant. The final immunization (100 µg of immunogen) was given without any adjuvant 3 days prior to cell fusion. The immunized splenocytes were isolated and fused with a hypoxanthine-aminopterine-thymidine (HAT) sensitive mouse myeloma cell line, P3-X63-Ag8-653, by the polyethylene glycol (PEG) method.23 Hybridoma producing MAb against berberine were cloned twice by the limited dilution method24 as follows: After centrifuging the cells from the wells of a 24 well plate that contains hybridomas, the cells were counted and suspensions with concentrations of 50, 10 and 5 cells ml−1 were prepared. Then 100 µl of a suspension of each concentration was added to each well of a 96 well tray. A week later, the plates were examined and screened for those wells containing only single colonies. Subsequently, cells were picked from positive wells and expanded in 24 well plate.The established hybridomas were scaled up and cultured in serum-free media (SFM), enriched RPMI1640-Dulbecco's-Ham's F12 medium (eRDF) supplemented with RD-1 additives containing 9 µg ml−1 of insulin, 20 µg ml−1 of transferrin, 20 µM of ethanolamine and 25 nM of sodium selenite.
Purification of MAb
MAb 1D5-3B-7 was purified using a protein G FF column (0.46 × 11 cm, Amersham Pharmacia Biotech, Uppsala, Sweden). The media containing the IgG was adjusted to pH 7 with 1 M Tris solution and loaded onto the column. After binding, the column was washed with 20 mM phosphate buffer (pH 7) and the adsorbed IgG was eluted with 100 mM of citrate buffer (pH 3). The eluted IgG was neutralized with 1 M Tris solution, then dialyzed against 5 changes of H2O for 3 days in 4 °C, and finally lyophilized.ELISA procedures
A 100 µl of berberine–HSA (1 µg ml−1 in 50 mM carbonate buffer, pH 9.6) was allowed to adsorb onto the wells of a 96 well flat-bottomed polystyrene immunoplate (NUNC, Roskilde, Denmark) for 1 h, and washed three times with washing buffer, PBS containing 0.05% Tween-20 (TPBS). The plate was treated with 300 µl of PBS containing 5% skimmed milk (SPBS) for 1 h to reduce nonspecific adsorption, and washed three times with TPBS and reacted with 100 µl of MAb for 1 h. The plate was washed three times with TPBS, then the MAb was combined with 100 µl of 1:1000 dilution of peroxidase-labelled anti-mouse IgG as the secondary antibody for 1 h. After washing the plate three times with TPBS, 100 µl of 100 mM citrate buffer (pH 4.0) containing 0.003% H2O2, and 0.3 mg ml−1 of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, ABTS (Wako Pure Chemical Ind. Ltd., Osaka, Japan) was added to each well, and the plate was incubated for 20 min. The absorbance at 405 nm was measured using an immunoplate reader (ImmunoMini NJ-2300, NUNC, Roskilde, Denmark). All reactions were carried out at 37 °C.Competitive ELISA was performed with almost the same protocol as direct ELISA except for one step. After blocking and washing, 50 µl of various concentrations of berberine or related compound solutions dissolved in 10% methanol were introduced into the wells of plate fixed berberine–HSA. An equal volume of MAb solution was added to the wells, and incubated for 1 h.
For data analysis, absorbance values from standards were mathematically fitted to a four-parameter logistic equation:
| y = {(A − D)/[1 + (x/C)B]} + D |
Quantitative analysis of berberine and its related compounds by HPLC
The HPLC system (Shimadzu, LC-10ADvp, Kyoto, Japan) was composed with dual pump, a Rheodyne 7725 injector, DGU-14A degasser and SPD-10Avp UV detector coupled to chromatopac C-R8A intergrator. The analysis was performed on Cosmosil column (4.6 id × 150 mm, 5C18-AR-II, Nacalai tesque, Kyoto, Japan) using a mobile phase composed of 0.1% HCOOH:CH3CN (85:15, v/v) at a flow rate 1.0 ml min−1. A 10 µl of aliquot of each sample was injected and monitored at 345 nm.Crude medicines, herbal medicines and sample preparation
Each 100.0 mg of powdered samples were sonicated in 3 ml of methanol for 30 min. The sample was centrifuged at 380 g for 5 min, and the supernatant was collected. The same procedure was repeated 4 times and the combined extract was adjusted to volume of 12 ml with methanol. The adjusted sample solution was filtered with 0.45 µm membrane and diluted serially with H2O for analysis.Results and discussion
Synthesis of hapten for conjugates
The specificity and selectivity of immunoassays are mainly determined by the antibody. Therefore, the design of hapten, target compound-like molecules containing a functional group suitable for protein conjugate, is the key step in the development of an immunoassay technique for small molecules. In the case of berberine (MW 366), it is too small to be immunogenic and must be conjugated to a large carrier protein that will function as the primary immunogen. In addition, berberine lacks a reactive group such as NH2, COOH, OH, SH, CO or CHO for linkage to a protein. To overcome this problem, 9-O-carboxymethyl berberrubine preserved the chemical structure of berberine was designed for the hapten and synthesized following Iwasa's method20 and Sadykov's method21 (see Fig. 1). The designed hapten and carrier protein were conjugated with a zero-length cross-linking procedure mediated by the water-soluble carbodiimide EDC to prevent the production of antibodies against the cross-linker bridge.25 The carbodiimide first reacts with carboxylic groups on 9-O-carboxymethyl berberrubine to form a highly active O-acylisourea intermediate and the activated carboxylic group then reacts with a primary amine to form an amide bond, with release of the EDC mediator as a soluble isourea derivative.Direct determination of hapten number in hapten–carrier protein conjugate by MALDI TOF MS
In order to confirm its immunogenicity, the hapten number in the berberine–carrier protein conjugate was determined by MALDI-TOF MS. Using experimental results and a molecular weight of 66433 for BSA, the calculated value of the berberine component (MW 366) was m/z 1022, indicating at least 3 molecules of berberine conjugated with BSA. This hapten number was estimated to be applicable for immunization. The number of berberine moieties contained in the berberine–HSA conjugate was determined, by its spectrum, to be approximately 4 molecules.Preparation of monoclonal antibody
Spleen cells (3.77 × 108) from the mouse immunized with berberine–BSA were hybridized with myeloma cells (7.74 × 107). The number of well-grown hybridomas was 109 strains. The cell fusion efficiency was 6.68% (109 wells/1632 wells). The titer of all supernatants was investigated by direct ELISA and 17 strains were selected. After scaling up for a week, 7 strains had high titers to berberine–BSA, but low titers to BSA. From the strains producing antibodies of high titer and grown more rapidly, three strains were cloned twice by the limited dilution method. Among 3 cloned strains, 1D5-3B-7 was chosen due to its reactivity and specificity for berberine. The clone 1D5-3B-7 was grown in eRDF media giving anti-berberine MAb.Characteristics of MAb 1D5-3B-7 against berberine
The reactivity of MAb 1D5-3B-7 against berberine was determined by a direct ELISA. The isotype of the secreted antibody was determined by using a mouse monoclonal antibody isotyping kit. MAb 1D5-3B-7 was classified as IgG1 that had k light chain. The reactivity of MAb was tested by direct ELISA using various concentrations (Fig. 2).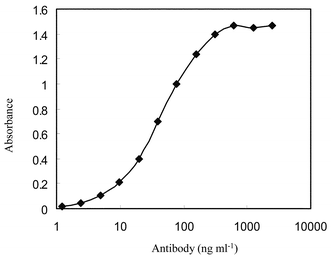 | ||
| Fig. 2 Reactivity of MAb 1D5-3B-7 against berberine–HSA. To examine the reactivity of the antibody, various concentrations of antibody were added to each well of an immunoplate coated with berberine-HSA (1 µg ml−1). | ||
Specificity and sensitivity of assay
Specificity of MAb 1D5-3B-7 was estimated by cross-reactivity measurement according to Weiler and Zenk's equation.26 The ELISA was not specific for berberine as showed in the cross-reactivity with structurally related compounds, coptisine, palmatine, but it did not react with other alkaloids (Table 1). This wide cross-reactivity is the major advantage of the antibody reagent used in this ELISA since berberine and structurally related alkaloids have the almost same pharmacological activity.27,28 These results show that the presence of charged residue in the berberine skeleton may be necessary as the epitope for MAb 1D5-3B-7. Furthermore, the phenol alkyl ether group in a molecule is also needed.| Cross-reactivity (%)a | |
|---|---|
| a Cross-reactivity (%) = [(quantity of berberine that inhibits antibody binding by 50%)/(quantity of cross-reactive compound that inhibits antibody binding by 50%)] × 100. The values are averages from triplicate determinations. b n.i. is <10% inhibition at the highest concentration (100 µg ml−1). | |
 Berberine 100 Berberine 100 |
 Coptisine 140.7 Coptisine 140.7 |
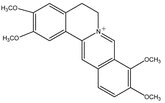 Palmatine 50.7 Palmatine 50.7 |
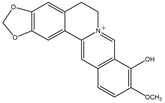 Berberrubine 15.1 Berberrubine 15.1 |
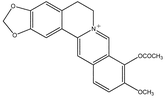 9-Acetylberberine 12.3 9-Acetylberberine 12.3 |
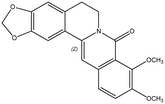 8-Oxoberbeine n.i.b 8-Oxoberbeine n.i.b |
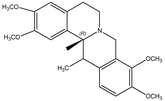 Corydaline n.i.b Corydaline n.i.b |
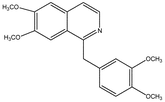 Papaverine n.i.b Papaverine n.i.b |
Various concentrations of berberine were incubated with MAb 1D5-3B-7 (80 ng ml−1) in an immunoplate adsorbed with berberine-HSA (1 µg ml−1). The calibration curve was obtained by averaging 12 individual standard curves performed in the course of berberine determination in samples. The linear range of the assay was extended from 1.56 to 50 µg ml−1 with the correlation coefficient (R2) of 0.9956. The detection limit was also estimated by IC90, the concentration of standard at which the initial binding was decreased by 10%. For berberine, the detection limit in this ELISA method was determined to be 780 ng ml−1.
Accuracy and variation of assay
In the standard curve for the competitive ELISA of berberine, the variations of repeatability from triplicate wells for each concentration (intra-assay) and the reproducibility from plate-to-plate (inter-assay) for four consecutive days were calculated by taking values in all aliquots of sample. Among ELISA runs, the coefficients of variation were below 15% inter-assay and below 4% intra-assay in most cases (data not shown). It becomes evident that intra-assay variations are lower than those of inter-assay. Although there are many factors such as coating step, the position of sample in immunoplate, multi-channel pipetter, edge effects due to evaporation, uneven temperature during incubation, and day-to-day variation, the results of accuracy and variation was acceptable.Recovery by competitive ELISA in spiked samples
6.25, 12.5 and 25 µg ml−1 of samples was spiked at the zero spiked level. The recovery was calculated by dividing the mean of all the obtained results using different ratios of added analyte, then multiplying by 100. As a result, the data ranged from 93.03 to 99.69% (data not shown).Comparison of ELISA and HPLC determination of berberine and its related compounds in medicinal plants
The correlation between the total concentration of berberine, coptisine and palmatine in crude extracts of individual plants determined by HPLC and ELISA was investigated (Table 2). Linear regression analysis of the plot gave acceptable results of which the equation was y = 0.9802x with correlation coefficients (R2) of 0.9383 (n = 4).| Crude plant | HPLC (mean ± SD, w/w, %) | ELISA (mean ± SD, w/w, %) | |||
|---|---|---|---|---|---|
| Berberine | Coptisine | Palmatine | Total | ||
| a A and B were classified according to their cultivated places. (A: China, B: Japan). b n.d. is not detected in the analysis. | |||||
| Coptidis rhizoma Aa | 7.48 ± 0.37 | 2.22 ± 0.11 | 1.76 ± 0.08 | 11.46 ± 0.56 | 11.94 ± 0.66 |
| Coptidis rhizoma Ba | 5.47 ± 0.22 | 1.34 ± 0.05 | 0.24 ± 0.01 | 7.05 ± 0.28 | 5.79 ± 0.34 |
| Hydrastis rhizoma | 5.54 ± 0.31 | n.d.b | n.d.b | 5.54 ± 0.31 | 5.11 ± 0.29 |
| Phellodendri cortex | 4.83 ± 0.23 | 0.11 ± 0.01 | 0.22 ± 0.00 | 5.16 ± 0.24 | 5.39 ± 0.24 |
Determination of the total concentration of berberine and its related compounds in various herbal medicines
After setting up assay validation and optimization, we determined the total concentration of berberine and related compounds in various herbal medicine samples by competitive ELISA. We concluded that it could be applied to the determination of the total concentration of berberine and related compounds in various herbal medicines (Table 3). Especially, it is an efficient analytical method for large scale certification in herbal medicines.| Sample | Prescription | Form | Composition ratio | ELISA (mean ± SD, w/w, %) |
|---|---|---|---|---|
| a n.d. is not detected in the analysis. | ||||
| 1 | Oren-gedoku-to | granule | 3.5/8.5 | 1.72 ± 0.08 |
| 2 | Oren-gedoku-to | capsule | 3/8 | 4.72 ± 0.21 |
| 3 | San'o-shashin-to | capsule | 1/3 | 12.65 ± 0.90 |
| 4 | San'o-shashin-to | granule | 3/9 | 9.54 ± 0.77 |
| 5 | Oren-to | granule | 3/24 | 1.02 ± 0.02 |
| 6 | Unsei-in | granule | 1/6 | 0.31 ± 0.09 |
| 7 | Shakuyaku-kanzo-to | granule | 0/18 | n.d.a |
In conclusion, the ELISA method described in this study can be applied as an analytical tool for determining the levels of berberine in commercial products, either as a single crude drug or in combination with other crude drugs, to establish one of the primary criteria for quality and conformity of herbal medicines. Although not perfectly suited for the analysis of crude drugs and traditional Chinese medicines, the sensitivity and selectivity of ELISA are sufficient to determine berberine and its related compounds which have similar pharmacological activities. To our knowledge, this is the first approach to immunoassay using MAb against berberine and its related compounds for the quantitative determination. We have been attempting to apply MAb for the eastern blotting procedure for identification of berberine type alkaloids in plant and animal tissue as previous reported29 and these results will be reported elsewhere.
Acknowledgements
This research in this paper was supported by the research fund of Kyushu University Foundation and Japan Kampo Medicine Manufacturers Association from Japan Kampo Medicine Manufacturers.References
- M. Sabir, M. H. Akhter and N. K. Bhide, Indian J. Physiol. Pharmacol., 1978, 22, 9–23 Search PubMed.
- N. Ivanovaska and S. Philipov, Int. J. Immunopharmacol., 1996, 18, 553–561 CrossRef.
- K. Iwasa, M. Moriyasu and B. Nader, Biosci. Biotechnol. Biochem., 2000, 64, 1998–2000 CAS.
- Y. H. Tai, J. F. Fesler, W. G. Marnane and J. F. Desjeux, Am. J. Physiol., 1981, 241, 253–258 Search PubMed.
- V. M. Mahajan, A. Sharma and A. Rattan, Sabouraudia, 1982, 20, 79–81 Search PubMed.
- J. L. Vennerstrom, J. K. Lovelace and V. B. Waits, Antimicrob. Agents Chemother, 1990, 34, 918–921 CAS.
- C. W. Lau, X. Q. Yao, Z. Y. Chen, W. H. Ko and Y. Huang, Cardiovasc. Drug Rev., 2001, 19, 234–244 Search PubMed.
- D. I. Kim, H. Pederson and C. K. Chin, J. Biotechnol., 1990, 16, 297–304 CrossRef CAS.
- A. K. Krey and F. E. Hahn, Science, 1969, 166, 755–757 CAS.
- V. Mikes and V. Dadak, Biochim. Biophys. Acta, 1983, 27, 231–239.
- S. El-Masry, M. A. Korany and A. H. Abou-Donia, J. Pharm. Sci., 1980, 69, 597–598 CAS.
- M. Govindan and G. Govindan, Fitoterapia, 2000, 71, 232–235 CrossRef CAS.
- C. M. Chen and H. C. Chang, J. Chromatogr., B, 1995, 665, 117–123 CrossRef CAS.
- E. A. Abourashed and I. A. Khan, J. Pharm. Sci., 2001, 90, 817–822 CrossRef CAS.
- H. M. Liebuch, R. Lehmann, C. Di Stefano, H. U. Häring, J. H. Kim and K. R. Kim, J. Chromatogr., A, 1998, 795, 388–393 CrossRef CAS.
- B. D. Hammock and R. O. Mumma, in Recent Advances in Pesticide Analytical Methodology, ed., J. Harvey and G. Zweig, American Chemical Society, Washington, 1980, pp 321–352 Search PubMed.
- A. Yoshida and Y. Takagaki, Nippon Rinsho, 1995, 53, 2316–2321 Search PubMed.
- M. P. Marco, S. Gee and B. D. Hammock, Trends Anal. Chem., 1995, 14, 415–425 CrossRef CAS.
- Y. Shoyama, H. Tanaka and N. Fukuda, Cytotechnology, 1999, 31, 9–27 CrossRef CAS.
- K. Iwasa and M. Kamigauchi, Phytochemistry, 1996, 41, 1511–1515 CrossRef CAS.
- Y. D. Sadykov, S. B. Davidyants, K. T. Poroshin and I. N. Grigina, Dokl. Akad. Nauk Tadzh. SSR, 1967, 10, 18–21 Search PubMed.
- Y. Shyoyama, R. Sakata, R. Isobe and H. Murakami, Org. Mass. Spectrom., 1993, 28, 987–988.
- G. Galfre and C. Milstein, Methods Enzymol., 1981, 73, 3–46 CAS.
- J. W. Goding, J. Immunol. Methods, 1980, 39, 285–308 CrossRef CAS.
- J. M. Tedder, A. Nechvatal and A. W. Murray, in Basic Organic Chemistry, John Wiley & Sons, 1972, ch. 6, pp 305–342 Search PubMed.
- E. W. Weiler and M. H. Zenk, Phytochemistry, 1976, 15, 1537–1545 CrossRef CAS.
- K. Iwasa, M. Moriyasu, T. Yamori, T. Turuo, D. U. Lee and W. Wiegrebe, J. Nat. Prod., 2001, 64, 896–898 CrossRef CAS.
- E. Kupeli, M. Kosar, E. Yesilada, K. H. C. Baser and C. Baser, Life Sci., 2002, 72, 645–647 CrossRef CAS.
- S. J. Shan, H. Tanaka and Y. Shoyama, Anal. Chem., 2001, 73, 5784–5790 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
