Comment on “Soxhlet extraction of acrylamide from potato chips” by J. R. Pedersen and J. O. Olsson, Analyst, 2003, 128, 332
Masaharu
Tanaka
,
Yukio
Yoneda
,
Yasuko
Terada
,
Eri
Endo
and
Toshihiro
Yamada
*
Food Safety Research Institute, Nissin Food Products Co., Ltd., Kusatsu, Shiga 525-0055, Japan. E-mail: t-yamada@mb1.nissinfoods.co.jp; Fax: 81 77 561 9199; Tel: 81 77 561 9117
First published on 12th December 2003
Recent findings, which showed acrylamide to be present in a range of fried and oven-cooked foods,1 raised a worldwide concern, because acrylamide is a neurotoxic and potential carcinogenic chemical in humans.2,3 Recent studies suggest that acrylamide can be formed in foods rich in carbohydrate and a specific amino acid, known as asparagine, during cooking at high temperatures.4–6 The Swedish findings were replicated in various countries and very similar results were obtained.7–9 The FDA has posted exploratory data indicating acrylamide levels in a limited number of US foods on the web sites.10 This data will be used to assess exposure of consumers to acrylamide and to determine the potential risk of acrylamide in foods to human health.Most of the methods used to analyze acrylamide in foods consist of similar steps including homogenization, solvent extraction, SPE column filtration, and derivatization of the extracted acrylamide, followed by mechanical detection such as GC-ECD, GC-MS, or LC-MS-MS.1,8,11,12 Since acrylamide is very soluble in water, many investigators have used water as an extraction solvent. For example, Rosén and Hellenäs12 have developed a method to measure acrylamide levels in foods by LC-MS-MS, in which water is added to the test sample prior to the SPE column filtration.
Recently, Pedersen and Olsson13 reported on a continuous extraction method using methanol as an extraction solvent and a modified Soxhlet extractor. They detected very high concentration of acrylamide (14500 µg kg−1) in one particular brand of potato chips. The result was about 7 times higher than previously reported concentrations, 2287 and 1993 µg kg−1, respectively obtained by two different methods. According to their data, complete extraction of acrylamide from potato chips required continuous treatment of the sample for about 7 days in a Soxhlet extractor. The potential impact of their method is very substantial because the FDA is going to assess the risk of acrylamide in foods using exploratory data obtained by commonly used techniques.
Here, we report some evidence for de novo formation of acrylamide in a Soxhlet extractor during the modified Soxhlet extraction procedure. Our data suggests that it is adequate to make use of the exploratory data posted by the FDA in risk assessments of acrylamide in foods.
Experiment
Materials and samples
Acrylamide was purchased from Tokyo Kasei Co. (98%, Extra pure grade; Tokyo Japan). Deuterium-labeled acrylamide-2,3,3-d3 was purchased from Cambridge Isotope Laboratories (98 atom % D; Andover, MA) and used as an internal standard. All other chemicals used were of guaranteed grade.Stock solutions of standards, 100 µg ml−1 of acrylamide or acrylamide-2,3,3-d3, were prepared in water and stored at 4 °C. Working standard solutions were prepared by mixing aliquots of acrylamide stock solution (equivalent to 0, 1, 3, 10, 30, 100 µg of acrylamide) and 100 µl of internal standard solution, and made up to 100 ml with water.
Potato chips, Mash Potato (both samples were made by a major Japanese manufacturer), and raw potato (Irish Cobbler; made in Nagasaki, Japan) were purchased at supermarkets in Shiga, Japan, from 14 July to 22 August 2003.
Sample treatment
Potato chips of mass 6.0 g were ground and defatted with 60 g n-hexane 3 times over. The weight decrease was 36.7%. A defatted 3.8 g sample was refluxed with 120 g methanol in a Soxhlet extractor (TOP®; Sougorikagaku Glass Products Co., Kyoto, Japan). A 200 ml boiling flask, with magnetic stirring, was heated at 65 °C, and was weighted daily to keep the solvent weight after 2 ml sampling. To obtain sample extract from the defatted potato chips, the mixture of defatted potato chips and 120 g methanol was refluxed in the boiling flask (without a Soxhlet siphon), and then the mixture was filtered on day 1, day 3, or day 5 of the continuous extraction period. Sample extract, i.e. the filtrate, was transferred into new flask and was refluxed for additional days.Freeze-dried and powdered samples (raw potato or Mash Potato, 6.0 g each) were refluxed with 120 g methanol in the same Soxhlet extractor as described above for 0 to 15 days.
Acrylamide in the sampled solutions was concentrated, purified, and analyzed by GC-MS after derivatization, according to the methods of the National Food Research Institute, Japan.9
GC-MS analysis
The derivatized samples in ethyl acetate were analyzed using a HP6890/ JMS SUN200 GC-MS (SIM mode), with the electron ionization source kept at 200 °C. The gas chromatograph was equipped with an electron pressure control system. The derivatized sample was chromatographed on a capillary DB-17MS column (0.25 mm id, 0.25 µm film, and 30 m length). Helium (99.9999% pure) was used as a carrier gas at a column flow rate of 1.0 ml min−1. One µl of the sample was injected by using the HP6890 automatic sampler/injector in a splitless mode. The injector temperature was 120 °C. The temperature program for the GC was as follows: isothermal for 1 min at 85 °C, a ramp rate of 25 °C min−1 to 175 °C, isothermal for 6 min, and a ramp rate of 40 °C min−1 to 250 °C.Determination of asparagine and sugar contents
The amount of free asparagine was analyzed by using High Speed Amino Acid Analyzer (Type 835; Hitachi High-Technologies Co., Tokyo, Japan) with 2619F column for physiological fluid determination (2.6 mm id, and 250 mm length; Hitachi) according to the manufacture's instructions. To examine reducing sugar contents, Agilent CE System (Agilent Technologies, Waldbronn, Germany) was used, and glucose, mannose, galactose, fructose, maltose, and lactose were analyzed using a standard fused silica capillary column (G1600-62211; Agilent Technologies).Results
Two Japanese laboratories have been publishing the data on acrylamide levels in various Japanese foods. The published values of acrylamide in potato chips were in the range of 439–3544 µg kg−1 by using LC-MS or LC-MS-MS methods.9,14 We obtained the value of 591 µg kg−1 from one particular brand of potato chips by using GC-MS methods. By applying the continuous extraction method and GC-MS analysis to the same potato chips, we found an amount of 3200 µg kg−1 in the original sample on day 15 of the treatment (Fig. 1). A constant concentration of acrylamide was reached in the extract on day 12. All these data were in good accordance with the previously published observations. | ||
| Fig. 1 Time-dependent change of acrylamide level in the methanol extract from defatted potato chips (3.8 g) by using the continuous extraction method. Samples were analyzed by GC-MS after derivatization, using acrylamide-2,3,3-d3 as the internal standard. The concentration of acrylamide in the extract is expressed as µg kg−1 in the original sample. | ||
Recently, it was confirmed by several groups that Maillard reactions involving asparagine could produce acrylamide in thermally processed foods.4,5 To confirm the possibility that free asparagine and reducing sugar, such as glucose, could be involved in Maillard reactions during reflux in Soxhlet extractor, we added L-asparagine (0.1 mmol) and D-(+)-glucose (0.1 mmol) into 80 g methanol with magnetic stirring, and the solution was refluxed. The flask was weighed daily and a 2 ml sample was subjected to GC-MS analysis. The acrylamide level in the methanol solutions was under the quantitation limit (10 µg kg−1) for 3 days, however, the acrylamide level became detectable even on day 1, and the amount of 156 µg kg−1 was obtained on day 15 (Fig. 2). Moreover, the mass spectrum of the derivatized acrylamide in the methanol solution was identical with that of the derivatized standard acrylamide (Fig. 3). This data indicates that free asparagine and reducing sugar, such as glucose, in methanol are able to generate acrylamide during reflux.
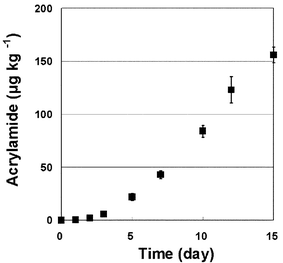 | ||
| Fig. 2 Concentration of acrylamide generated newly in 80 g methanol by Maillard reaction involving D-(+)-glucose (0.1 mmol) and L-asparagine (0.1 mmol). Samples were analyzed by GC-MS after derivatization, using acrylamide-2,3,3-d3 as the internal standard. Error bars represent standard deviations (n = 3). | ||
 | ||
| Fig. 3 Mass spectrum of 2,3-dibromopropaneamide (2,3-DBPA), upper: sampled solution containing acrylamide (309 µg kg−1) generated from the mixture of D-glucose and L-asparagine, lower: standard solution of acrylamide (300 µg kg−1). | ||
Next, we analyzed free asparagine in the sample extracts from the defatted potato chips on day 1, 3, and 5 of the treatment (Fig. 4a). The asparagine levels in the sample extracts were found to be in the same range (1570–1840 mg kg−1). When the sample extracts were refluxed, the acrylamide levels in the sample extracts were gradually increased (Fig. 4b). On the other hand, the asparagine levels in the sample extracts were decreased by the same treatment. This data suggests that defatted potato chips could supply free asparagine and reducing sugar to the sample extract, and that the free asparagine and reducing sugar were consumed by the Maillard reaction during reflux.
 | ||
| Fig. 4 a: Time-dependent changes of asparagine concentrations in the sample extracts from the defatted potato chips on day 1 (circles), 3 (squares), and 5 (triangles) of the treatment. The sample extracts were transferred into new flask and were refluxed for additional days. The concentration of acrylamide in the extract is expressed as mg kg−1 in the original sample. b: Time-dependent changes of acrylamide concentrations in the sample extracts from the defatted potato chips. The concentration of acrylamide in the extract is expressed as µg kg−1 in the original sample. Error bars represent standard deviations (n = 3). | ||
To confirm whether the de novo formation of acrylamide in a Soxhlet extractor could occur, freeze dried and powdered raw potato was refluxed with 120 g methanol in a Soxhlet extractor. Since acrylamide has not been detected in unheated control foods or boiled foods,1,9 it was expected that this treatment would not extract any acrylamide from the sample promptly, and indeed, in accordance with previous observations, we detected no acrylamide on day 0 of the treatment. Surprisingly, however, we detected an enormous amount (6887 µg kg−1) of acrylamide in methanol on day 7 of the treatment (Fig. 5). This data clearly demonstrates the existence of de novo formation of acrylamide in a Soxhlet extractor.
 | ||
| Fig. 5 Time-dependent change of acrylamide level in the methanol extract from freeze-dried raw potato (6.0 g) by using the continuous extraction method. Samples were analyzed by GC-MS after derivatization, using acrylamide-2,3,3-d3 as the internal standard. The concentration of acrylamide in the extract is expressed as µg kg−1 in the original sample. Error bars represent standard deviations (n = 3). | ||
Discussion
Levels of acrylamide recovered from food samples were reported to vary to a large extent among food types.9,14 In our laboratory, acrylamide recovered from potato chips was typically 95.1 ± 2.6%. Moreover, the corrected recovery level for potato chips was 99.9 ± 2.0%, according to our internal standard, which suggests that the method would perform well in the analysis of acrylamide in potato chips. Although the continuous extraction method was designed to achieve complete extraction of acrylamide from food samples,13de novo formation of acrylamide in a Soxhlet extractor, rather than complete extraction of acrylamide, was observed during the process (Fig. 5). Therefore the continuous extraction method should not be applied for the analysis of acrylamide in thermally processed foods.As mentioned above, it was confirmed recently that Maillard reactions involving asparagine could produce acrylamide in thermally processed foods;4,5 and acrylamide formation from its precursors, such as decarboxylated Amadori product, is believed to require relatively high temperatures.6 However, our data suggest that free asparagine and reducing sugar in methanol are able to generate acrylamide at a relatively low temperature, i.e. not more than 65 °C (Fig. 1). It is not known whether the same reactions involving asparagine and reducing sugar in foods could occur during cooking at relatively low temperatures. However, our data indicate that, if specific conditions (as yet unknown) were in place, acrylamide might be formed to a much greater extent than previously we thought in foods cooked at relatively low temperatures.
Pedersen and Olsson reported that a constant concentration of acrylamide was reached in the methanol extract on day 7 of the continuous extraction.13 We reported here that a constant concentration of acrylamide was reached in the methanol extract on day 12. This difference would be explained by the difference in usage of deactivated glass beads, which were used by Pedersen and Olsson to minimize the dead volume of thimbles. However, it is unclear why a constant concentration of acrylamide was reached when it was. In fact, we found enough free asparagine and reducing sugar for acrylamide formation in the methanol extract even on day 12 of the treatment (data not shown). If this is true, what mechanism could suppress the formation of acrylamide in the methanol solution during reflux? One possible mechanism is that Maillard reactions involving amino acids other than asparagine and reducing sugar might compete with the acrylamide formation. Evidently, we could not observe a constant concentration of acrylamide in the methanol solution, when the solution was refluxed with additions of asparagine and glucose as reactants for 15 days (Fig. 2). It is surely important to investigate such competitors for acrylamide formation in order to control the formation of acrylamide in foods.
References
- E. Tareke, P. Rydberg, P. Karlsson, S. Eriksson and M. Törnqvist, J. Agric. Food Chem., 2002, 50, 4998–5006 CrossRef CAS.
- IARC, Some Industrial Chemicals, 1994, vol. 60, pp. 389–433 Search PubMed.
- FAO/WHO, Health Implications of Acrylamide in Food (Geneva: WHO), 2002, pp. 8–9 Search PubMed.
- D. S. Motram, B. L. Wedzicha and A. T. Dodson, Nature, 2002, 419, 448–449 CrossRef CAS.
- R. H. Stadler, I. Blank, N. Varga, F. Robert, J. Hau, P. Guy, M.-C. Robert and S. Riediker, Nature, 2002, 419, 449–450 CrossRef.
- V. A. Yaylayan, A. Wnorowski and C. P. Locas, J. Agric. Food Chem., 2003, 51, 1753–1757 CrossRef CAS.
- Websites: http://www.acrylamide.iit.edu/LinksPage.htm, http://www.snt.no/nytt/tema/Akrylamid/analyse_eng.html.
- J. S. Ahn, L. Castle, D. B. Clarke, A. S. Lloyd, M. R. Philo and D. R. Speck, Food Addit. Contam., 2002, 19, 1116–1124 CrossRef CAS.
- H. Ono, Y. Chuda, M. Ohnishi-Kameyama, H. Yada, M. Ishizaka, H. Kobayashi and M. Yoshida, Food Addit. Contam., 2003, 20, 215–220 CAS.
- The FDA websites: http://www.cfsan.fda.gov/∼dms/acrydata.html, http://www.cfsan.fda.gov/∼dms/acrydat2.html (2003 update).
- A. Hashimoto, Analyst, 1976, 101, 932–938 RSC.
- J. Rosén and K.-E. Hellenäs, Analyst, 2002, 127, 880–882 Search PubMed.
- J. R. Pedersen and J. O. Olsson, Analyst, 2003, 128, 332–334 RSC.
- S. Takatsuki, S. Nemoto, K. Sasaki and T. Maitani, J. Food Hyg. Soc. Japan, 2003, 44, 89–95 Search PubMed.
| This journal is © The Royal Society of Chemistry 2004 |
