Comment on “Soxhlet extraction of acrylamide from potato chips” by J. R. Pedersen and J. O. Olsson, Analyst, 2003, 128, 332
Jonathan W.
DeVries
and
Brett E.
Post
*
General Mills, 9000 Plymouth Ave. N, Golden Valley, MN, USA. E-mail: Brett.Post@genmills.com; Fax: 763 764 2563; Tel: 763 764 2518
First published on 12th December 2003
A recent publication by J. R. Peterson and J. O. Olsson1 concluded that the current, generally accepted practices2,3 of extracting acrylamide from foods for the purpose of analysis are inadequate. The methods typically use a mechanically assisted extraction into a solvent, followed by several clean up steps, and finally quantitation by LC-MS/MS or GC-MS. The method used in this investigation4 is similar to that described by Rosén and Hellenäs.2 All the cited methods provide similar results for a given sample. Peterson and Olsson1 go on to describe and recommend a procedure using Soxhlet apparatus to extract fried and baked foods using methanol as the extraction solvent. Use of this procedure resulted in up to a five-fold increase in apparent acrylamide content over previously published values for similar foods. The authors1 attributed the increase to a more complete and rigorous extraction of acrylamide from the food samples. Investigations in our laboratories were carried out to determine if the increased apparent acrylamide level was due to more rigorous extraction or rather due to thermally induced generation of additional acrylamide using the Soxhlet extraction procedure described.Experimental
Sample procurement
Russet Burbank Idaho potatoes were purchased at a local supermarket. Asparagine, glucose, and acrylamide were supplied by Sigma-Aldrich in St. Louis, MO. Cambridge Isotope Laboratories in Andover, MA supplied 2,3,3-d3-acrylamide. Reagent methanol was purchased from VWR International in West Chester, PA.Sample preparation
Raw Russet Burbank Idaho potato was peeled and cut into approximately 1 cm × 1 cm pieces. About 10 g was placed into a steel cup and covered with liquid nitrogen until thoroughly frozen. The cup was then placed onto a Stein mill and the potato pieces were ground to a fine powder. The final appearance was that of white flour. Microscopic examination of the resulting potato flour assured that cell disruption had occurred, thus ensuring that any free asparagine and/or glucose within cells would be available for potential extraction. Since cell disruption also occurs when cooking at temperatures high enough to gelatinize starch, raw potato was boiled in tap water until it was cooked sufficiently such that a fork could be inserted into the center with almost no force. Free water was drained from the cooked potato prior to homogenization in a food blender.Reflux experiments
Refluxing apparatus consisting of a 250 mL boiling flasks with a water-cooled condenser open to the atmosphere were assembled. Heat was transferred to the flask via a heating mantle controlled by a continuously variable transformer. Two different quantities of asparagine and two different mixtures of glucose and asparagine were placed directly into the boiling flask and refluxed for 16 h at a controlled drip rate of 1 drip s−1. Table 1 shows the results of this exercise.| R1b | R2c | R3d | R4e | |
|---|---|---|---|---|
| a All reflux experiments ran for 16 h. b R1 consisted of 0.3034 g asparagine refluxed in 100 mL methanol. c R2 consisted of 1.0003 g asparagine refluxed in 100 mL methanol. d R3 consisted of 0.3011 g asparagine + 0.3049 g glucose refluxed in 100mL methanol. e R4 consisted of 1.0045 g asparagine + 0.1047 g glucose refluxed in 100mL methanol. | ||||
| Acrylamide/ng | 1104 | 2989 | 79123 | 94483 |
Soxhlet extraction
The Soxhlet extraction apparatus used consisted of 250 mL round bottom flasks and 125 mL extractor bodies with condensers open to the atmosphere. Heat was transferred to the flask via a heating mantle controlled by a continuously variable transformer. Samples were placed into 25 × 80 mm cellulose extraction thimbles, and the Soxhlet extraction was carried out using 100 mL of reagent grade methanol heated sufficiently to produce approximately one drop per second from the condenser. Four samples, asparagine and glucose mixtures at two different ratios, the potato flour and the boiled potato were extracted by this method for up to ten days. At various time intervals as noted in the tables and figures, a 10 mL sample of the methanol was removed for analysis. The quantity of removed methanol was replaced with fresh methanol to maintain a solvent volume of 100 mL in the extraction apparatus. Figs. 1–4 illustrate the results this sub sampling.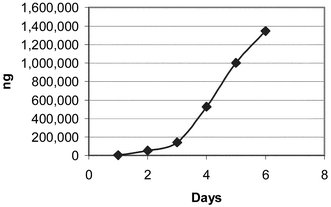 | ||
| Fig. 1 Glucose 0.3 g + asparagine 0.1 g S1. | ||
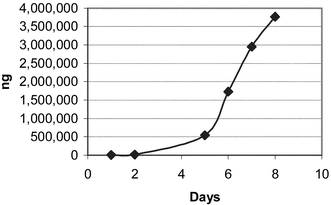 | ||
| Fig. 2 Glucose 0.3 g + asparagine 0.3 g S2. | ||
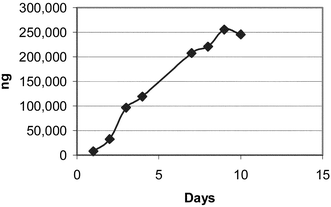 | ||
| Fig. 3 Raw potato S3. | ||
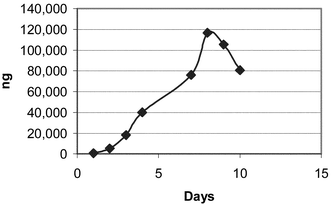 | ||
| Fig. 4 Boiled potato S4. | ||
To establish that no acrylamide was present in the raw or boiled potato, or in commercially available asparagine and glucose, portions of each were analyzed prior to the start of the experiment. The potato flour and the boiled potato were analyzed for free asparagine and free glucose as well to establish a baseline quantity of the potential reactants present in the samples. The results of all these tests can be seen in Table 2.
| Methanol in boiling flaskf | S1b | S2c | S3d | S4e |
|---|---|---|---|---|
| a Results of all determinations performed initially (time 0), and at the final time (indicated for each sample below). b S1 consisted of 0.3074 g glucose + 0.1008 g asparagine and had a final time of 144 h. c S2 consisted of 0.3009 g glucose + 0.3018 g asparagine and had a final time of 192 h. d S3 consisted of potato flour weighing 6045.1 mg and had a final time of 240 h. e S4 consisted of boiled potato weighing 6005.8 mg and had a final time of 240 h. f 100 mL of methanol was placed in each system and maintained throughout the extraction. g This value is at the final time of 240 h. The peak for this particular sample occurred at 192 h with a value of 0.12 mg. | ||||
| Final acrylamide/mg | 1.35 | 3.76 | 0.25 | 0.08g |
| Final asparagine/mg | 13.2 | 139.9 | 7.6 | 8.5 |
| Final glucose/mg | 46.9 | 54.7 | <1.5 | <1.5 |
Determinations
Results and discussion
Table 1 shows the results of the reflux experiments. Boiling asparagine in methanol does generate acrylamide even in the absence of a reducing sugar, however the presence of glucose speeds the process significantly. This also proved to be the case in the Soxhlet extraction systems as shown in Table 2.Table 2 shows the results of determinations performed on samples when they were placed into the Soxhlet system and when they were removed. It was not considered necessary to test the reagent grade methanol initially. The decrease in the initial quantity of the two reactive analytes of interest in the thimble and their presence in the methanol in the boiling flask after the extraction is complete, demonstrates that asparagine and glucose are being transferred by the refluxing methanol from the Soxhlet thimble to the boiling flask. Once the two compounds are in the boiling flask they are reacting in the boiling methanol to form acrylamide as demonstrated by the reflux reactions. Figs. 1–4 illustrate the generation of acrylamide observed in the sub samples of methanol analyzed during the extractions.
There was not sufficient residue in the thimbles after the extractions to perform both final asparagine and final glucose analyses. Asparagine analysis was selected because its lower solubility in methanol7 made it the more likely constituent of the residue. Glucose is soluble at 1 g per 60 mL of methanol,7 reasonably ensuring that all the glucose was extracted from the thimbles. In fact, there was no visible residue in the thimble that had contained mixture S1.
Sixteen percent of the asparagine placed in thimble S2 was still in the thimble after 192 h of continuous extraction, demonstrating the effect of the low solubility of asparagine in methanol. All of the asparagine and glucose originally in the thimble cannot be accounted for as either generated acrylamide or residue in the thimble or dissolved in the methanol after the extraction. Simultaneous reactions that also consume glucose and asparagine are likely occurring.
Table 3 shows the results of the acrylamide determinations. Figs. 1–4 illustrate the Soxhlet portion of this data graphically. Figs. 1 and 2 show a lag in production during days 1 and 2. The solubility and dissolution rate of the asparagine by the methanol may be responsible for this effect. A possible explanation is that the glucose likely went into solution during the first several hours, but the asparagine may not have been present in solution in a large enough concentration for significant acrylamide production until after day 2. Neither the asparagine nor glucose appears to have become limiting reagents in the case of Mixtures S1 and S2. (Table 2). After 6 and 8 days respectively, the level of acrylamide was still rising (Figs. 1 and 2) and measurable levels of both asparagine and glucose remained in the methanol. On the other hand glucose appears to be the limiting reagent in the case of the potato samples S3 and S4. Figs. 3 and 4 both peak around day 8 or 9 with a decrease in acrylamide thereafter as the acrylamide decomposes. Acrylamide decomposition tends to occur after long periods of increased temperature.8,9 For the potato samples, the acrylamide formation curves are more linear from the beginning. This may be due to asparagine and glucose having to be extracted from the starchy matrix of the potato, leading to the slower but steady production of acrylamide.
| Sample and sample mass | Hours | Method | Acrylamide produced/ng |
|---|---|---|---|
| Asparagine 0.3034 g | 16 | Reflux | 1104 |
| Asparagine 1.0003 g | 16 | Reflux | 2989 |
| Glucose 0.3049 g + asparagine 0.3011 g | 16 | Reflux | 79123 |
| Glucose 0.1047 g + asparagine 1.0045 g | 16 | Reflux | 94483 |
| Raw potato 6.0451 g | 24 | Soxhlet | 8204 |
| Raw potato 6.0451 g | 48 | Soxhlet | 32608 |
| Raw potato 6.0451 g | 72 | Soxhlet | 96754 |
| Raw potato 6.0451 g | 96 | Soxhlet | 119513 |
| Raw potato 6.0451 g | 168 | Soxhlet | 207481 |
| Raw potato 6.0451 g | 192 | Soxhlet | 221116 |
| Raw potato 6.0451 g | 216 | Soxhlet | 255635 |
| Raw potato 6.0451 g | 240 | Soxhlet | 245759 |
| Boiled potato 6.0058 g | 24 | Soxhlet | 799 |
| Boiled potato 6.0058 g | 48 | Soxhlet | 5365 |
| Boiled potato 6.0058 g | 72 | Soxhlet | 18269 |
| Boiled potato 6.0058 g | 96 | Soxhlet | 39962 |
| Boiled potato 6.0058 g | 168 | Soxhlet | 76066 |
| Boiled potato 6.0058 g | 192 | Soxhlet | 116591 |
| Boiled potato 6.0058 g | 216 | Soxhlet | 105388 |
| Boiled potato 6.0058 g | 240 | Soxhlet | 80594 |
| Glucose 0.3074 g + asparagine 0.1008 g | 24 | Soxhlet | 4376 |
| Glucose 0.3074 g + asparagine 0.1008 g | 48 | Soxhlet | 53929 |
| Glucose 0.3074 g + asparagine 0.1008 g | 72 | Soxhlet | 141158 |
| Glucose 0.3074 g + asparagine 0.1008 g | 96 | Soxhlet | 526173 |
| Glucose 0.3074 g + asparagine 0.1008 g | 120 | Soxhlet | 1001326 |
| Glucose 0.3074 g + asparagine 0.1008 g | 144 | Soxhlet | 1345723 |
| Glucose 0.3009 g + asparagine 0.3018 g | 24 | Soxhlet | 3633 |
| Glucose 0.3009 g + asparagine 0.3018 g | 48 | Soxhlet | 16962 |
| Glucose 0.3009 g + asparagine 0.3018 g | 120 | Soxhlet | 539941 |
| Glucose 0.3009 g + asparagine 0.3018 g | 144 | Soxhlet | 1724191 |
| Glucose 0.3009 g + asparagine 0.3018 g | 168 | Soxhlet | 2948780 |
| Glucose 0.3009 g + asparagine 0.3018 g | 192 | Soxhlet | 376471 |
Contrary to the conclusion of Peterson and Olsson that current extraction methods are not sufficiently aggressive when analyzing for acrylamide, it appears the aggressive conditions chosen by Peterson and Olsson are sufficient to generate significant quantities of acrylamide from pure standards and boiled, and raw potatoes shown previously to contain no measurable levels of acrylamide.
Conclusion
While analytical methodology can often be improved upon, the currently accepted practices for extracting acrylamide from foods extract the analyte while avoiding conditions that actually generate acrylamide during the analysis. The experimental work done in our laboratory demonstrates that significant quantities of acrylamide are generated during methanolic Soxhlet extraction of potatoes and pure glucose/asparagine mixtures. While striving to produce optimum results through method improvement is always a goal of analytical chemists, Soxhlet extraction with methanol is not a viable alternative to mechanical extraction in the case of acrylamide analysis. Inaccuracies created by thermally induced acrylamide generation override any benefit gained as a result of more rigorous extraction.References
- J. O. Pedersen and J. O. Olsson, Analyst, 2003, 128, 332–334 RSC.
- J. Rosén and K. E. Hellenäs, Analyst, 2002, 178, 880–882 Search PubMed.
- E. Tareke, P. Rydberg, P. Karlsson, S. Eriksson and M. Törnqvist, J. Agric. Food Chem., 2002, 50, 4998–5006 CrossRef CAS.
- S. M. Musser, USFDA draft method available at http://www.cfsan.fda.gov/∼dms/acrylami.html.
- J. Hippe, Potato Res., 1988, 31, 535–540 Search PubMed.
- The Merck Index, 10th Edition, s.v. “asparagine” and “glucose”.
- Official Methods of Analysis of AOAC International, 17th Edition, AOAC Official Method 977.20..
- M. Biedermann, S. Biedermann-Brem, A. Noti and K. Grob, Official Food Control Authority of the Canton of Zürich, Switzerland, private communication.
- D. S. Mottram, B. L. Wedzicha and A. T. Dodson, Nature, 2002, 419, 488 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
