Analysis of erythromycin and tylosin in bovine muscle using disposable screen printed electrodes
Nagwa H. S. Ammidaa, G. Volpeb, R. Draiscic, F. delli Quadric, L. Palleschic and G. Palleschi*b
aDepartment of Chemistry, Garyounis University, Benghazi, Libya
bDipartimento di Scienze e Tecnologie Chimiche, Università Tor Vergata, via della Ricerca Scientifica, 00133 Roma, Italy
cLab. Medicina Veterinaria, Istituto Superiore di Sanità, V.le Regina Elena n.299, 00161 Roma, Italy
First published on 5th December 2003
Abstract
A disposable electrochemical enzyme-linked immunosorbent assay (ELISA) for the detection of two macrolides (erythromycin and tylosin) in bovine muscle was developed using a screen printed electrode (SPE) system as a differential pulse voltammetry (DPV) transducer with mouse anti-erythromycin (and anti-tylosin) monoclonal antibodies (MAb) serving as molecular recognition elements. The immunochemical system makes use of the competition assay principle, and employs an erythromycin (or tylosin)–BSA conjugate as coating molecule. After competition between free and coated analyte for the antibodies, the activity of the alkaline phosphatase labelled antiglobulins was measured electrochemically using 1-naphthylphosphate as substrate. Using standard solutions of erythromycin and tylosin, the detection limit of the assay was 0.2 ng mL−1 determined to be for erythromycin and 2.0 ng mL−1 for tylosin, while the sensitivity (25% inhibition concentration) was 1.0 ng mL−1 for erythromycin and 3.0 ng mL−1 for tylosin. The suitability of the assay for quantification of erythromycin and tylosin in bovine muscle was also studied. Spiked and real samples were analysed using the immunosensor system developed here. The ELISA showed precision values (relative standard deviation, RSD%) ranging from 4 to 9% for erythromycin and from 8 to 15% for tylosin; the accuracy (relative error, RE%) ranged from −11 to 6% and from −4 to 12% for erythromycin and tylosin, respectively. Results obtained on real samples were confirmed by micro-liquid chromatography coupled on line with tandem mass spectrometry (micro-LC-MS-MS), using an atmospheric pressure ionisation (API) source and an ionspray (IS) interface. The latter provides unequivocal identification and quantification of the analytes at the level of interest.
Introduction
Erythromycin and tylosin are two macrolide antibiotics that are isolated from Streptomyces spp. and used for the treatment of bacterial infections caused by Gram-positive organisms. These two macrolides are widely used in veterinary medicine to treat respiratory diseases and enteric infections in cattle, sheep, swine and poultry. Specifically, tylosin is used to reduce the incidence of liver abscesses in feedlot cattle. Erythromycin and tylosin are also used as feed and water additives for growth promotion or disease prophylaxis.1–3 Incorrect use of these antibiotics may leave residues in edible tissues, thus causing toxic effects for consumers, e.g. allergic reactions in hypersensitive individuals or indirectly, problems resulting from the induction of resistant strains of bacteria.4For the protection of the consumer, maximum residue limits (MRLs) have been established for these antibiotics according to European Union (EU) regulations. For bovine muscles, MRLs are set at 100 µg kg−1 and 200 µg kg−1 for tylosin and erythromycin, respectively. In addition, measuring techniques to monitor certain pharmacological substances and residues in live animals and animal products have been established by the EU with Council Directive 96/23/EEC.5
Several papers report that erythromycin and tylosin have been determined in raw materials, pharmaceutical dosage forms, and in biological samples such as blood plasma, serum, urine and tissues by microbiological assay6–8 and radioimmunoassay.9 The first method is neither specific nor sensitive enough for the detection of the MRLs established by regulatory authorities; the latter, on the other hand, allows rapid, sensitive and specific screening of a large number of samples. However, the major disadvantage of this conventional technique is that it requires radioisotopes and scintillation fluids.
In a review written by Kanfer et al.,10 applications of high-performance liquid chromatography coupled with different detectors for microlide determination are reported.
Gas chromatography and liquid chromatography coupled to mass spectrometry, on account of their high specificity based on the structural information of the analytes, have also been reported as useful confirmatory methods for the determination of macrolides in animal tissues.11–14 However, the analysis of these compounds with chromatographic methods is time consuming and equipment costs are high.
Recently, an enzyme-linked immunosorbent assay (ELISA) for the detection of erythromycin and tylosin in bovine muscle has been developed by our group.15 It uses mouse anti-erythromycin and anti-tylosin MAb, horseradish peroxidase as enzyme label, and an electrochemical flow injection analysis (FIA) system for signal detection.
The objective of the present study was the development of disposable immunosensors, based on the ELISA principle, for a simple and fast measurement of nanogram amounts of erythromycin and tylosin in bovine muscle. The ELISA is performed in a competitive format in which erythromycin (or tylosin) and anti-erythromycin (or anti-tylosin) MAb, are allowed to react immunologically with erythomycin–BSA (or tylosin–BSA) conjugate that are immobilised on the surface of the SPEs. After washing off the excess materials, the amount of bound antibody is evaluated using antiglobulins linked with alkaline phosphatase (Ab2–AP) specific for the former. The activity of the enzyme was measured using 1-naphthylphosphate as substrate and DPV as electrochemical technique. The current response is inversely proportional to the amount of macrolide antibiotic present in the sample.
In this study micro-LC-MS-MS, using an API source and an IS interface, was employed as confirmatory method.12 The results obtained show that disposable SPEs for performing electrochemical ELISA are suitable as a rapid screening method for the assessment of erythromycin and tylosin in bovine muscle.
Experimental
Reagents and materials
The monoclonal antibodies (anti-erythromycin and anti-tylosin) and conjugates (erythromycin–BSA and tylosin–BSA) were purchased from Biodesign International (Saco, ME, USA). Purified alkaline phosphatase-labelled goat anti-mouse IgG (H+L) was from Bio-Rad Laboratories (Hercules, CA, USA). Diethanolamine and 1-naphthylphosphate were from Fluka (Gallarate, Milan, Italy). Erythromycin, tylosin, roxithromycin, phosphate-buffered saline (PBS), polyvinylalcohol (PVA), polyoxyethylene-sorbitan monolaurate (Tween 20) and all other reagents were obtained from Sigma (St. Louis, MO, USA).Ultra-pure water was produce with a Milli-Q system (Millipore, Bedford, MA, USA).
Apparatus
All DPV measurements were performed using a computer controlled polarographic analyser, model 433 A (Amel, Milan, Italy). SPEs used for DPV measurements were printed with a high performance multi-purpose precision screen printer DEK 245 (DEK, Weymouth, UK). Inks were from Acheson Italia (Milan, Italy). The electrodes were printed according to a procedure that had been optimised in the Biosensors Laboratory of the University of Florence (Italy).16 The device, which consists of a graphite working electrode, a silver pseudo-reference electrode, and a graphite counter electrode, forms a complete electrochemical cell. The diameter of the working electrode was 0.3 cm which resulted in an apparent geometric area of 0.07 cm2.Chromatographic separations were performed under isocratic conditions using a reversed phase LC-ABZ Supelcosil microbore column (Fallavier, Saint Quentin, France) (300 × 1 mm id, 5 µm film thickness) at room temperature, with a mobile phase of acetonitrile–methanol–1% trifluoroacetic acid (60 + 20 + 20 v/v/v) and a flow rate of 50 µL min−1.
Mass spectral analyses were performed on a Perkin-Elmer SCIEX (Thornhill, Ontario, Canada) API III Plus triple-quadrupole mass spectrometer equipped with an API source and an IS interface set at a voltage of 5500 V.
Procedures
One Step Competition:
Equal volumes of anti-erythromycin MAb (diluted 1:100 in PBS) and erythromycin standard solutions (prepared in PBS or in a blank of bovine muscle extract) were combined. Then 6 µL of this mixture was added to the working electrode surface and allowed to react for 30 min.
Two Step Competition:
In the first step, equal volumes of anti-tylosin MAb (diluted 1:25 in PBS) and standard tylosin solutions (prepared in PBS) were allowed to react at room temperature for 10 min. In the second step, 6 µL of this mixture was placed on the surface of the working SPEs and the competition was performed for 30 min.
The same procedure was used when the competition was performed in bovine muscle extract. In this case, a mixture of anti-tylosin MAb (diluted 1:15 in PBS) and standard tylosin solutions (prepared in a blank of bovine muscle extract) was used.
For both one and two step competition, the labelling step was performed by adding 6 µL of the Ab2-AP, diluted 1:100 in PBS, and incubating for 15 min.
Between each step (coating, blocking, competition and labelling) a three cycle washing procedure (two washings using 0.05% Tween 20 in PBS and then one with PBS) was adopted.
The antibody macrolide complex was revealed by adding 100 µL of substrate solution (1mg mL−1 of 1-naphthylphosphate in 0.97 M diethanolamine buffer (DEA), pH 9.8, + 1 mM MgCl2 + 0.15 M KCl) to each electrode. After 2 min of incubation, the current response was measured with DPV.
All data points were carried out in duplicate and each value was the average of two determinations.
DPV measurements
All DPV measurements were performed in the potential range 0 to +600 mV vs. a silver screen printed pseudo-reference electrode, with a pulse width of 60 ms, a pulse height of 50 mV and a scan speed of 100 mV s−1.17Roxithromycin was used as an internal standard. As to operative conditions, the orifice potential voltage (OR) was set at 80 V for erythromycin, at 50 V for tylosin and at 60 V for roxithromycin. The protonated molecules, [M + H]+, at m/z 734, 916 and 837 for erythromycin, tylosin and roxithromycin, respectively, were the precursor ions for collision-induced dissociation (CID) and two diagnostic product ions for each analyte were identified, in order to carry out selected reaction monitoring (SRM) LC-MS-MS analyses. These were implemented using the double precursor–product ion combinations of m/z 734 → 158 and m/z 734 → 576 for erythromycin, m/z 916 → 174 and m/z 916 → 772 for tylosin, m/z 837 → 158 and m/z 837 → 679 for roxithromycin (internal standard).
Sample extraction
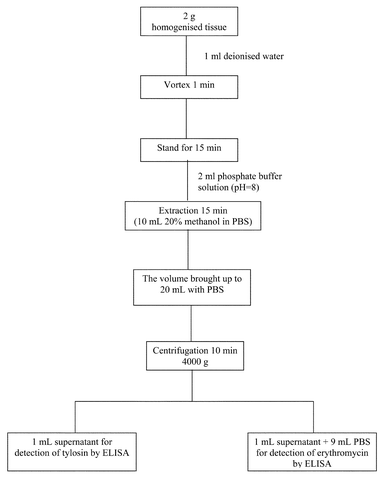 | ||
| Fig. 1 Modifying extraction procedure for erythromycin and tylosin in bovine tissue. | ||
Results and discussion
Optimisation of ELISA parameters
ELISA parameters such as dilution of antibodies and conjugates were initially optimised using standard solutions of the analytes under investigation.Different dilutions of specific antibodies and conjugates were tested in order to establish the best conditions for the competitive ELISA. The titration curves of anti-erythromycin (or anti-tylosin) MAb and erythromycin-BSA (or tylosin-BSA) conjugate are shown in Fig. 2. In order to perform these experiments, 6 µL of varying dilutions of anti-erythromycin (or anti-tylosin) antibodies were allowed to react (for 30 min) with different dilutions of erythromycin–BSA (or tylosin–BSA) conjugate that had been previously coated on the electrode surfaces. The procedures adopted for the immobilisation, blocking and washing steps were those reported in the Experimental section. As shown in Fig. 2, the best conditions were obtained using a dilution of 1:50 for erythromycin–BSA conjugate, while an insignificant difference between 1:20 and 1:10 dilutions was observed for tylosin–BSA conjugates. Therefore dilutions of 1:50 and 1:20 were chosen for erythromycin–BSA and tylosin–BSA conjugates respectively. Dilutions of 1:200 and 1:50 were selected for anti-erythromycinn MAb and anti-tylosin MAb respectively. These antibody dilutions represent a compromise between the maximum bound signals and the inflection points of the titration curves.
![Titration curves: (a) different dilutions of erythromycin–BSA conjugate [1:50 (●), 1:100 (Δ) and 1:200 (◆)] with different dilutions of anti-erythromycin MAb. (b) different dilutions of tylosin–BSA conjugate [1:10 (Δ), 1:20 (●) and 1:50 (◆)] with different dilutions of anti-tylosin MAb. The current response, detected with screen printed electrodes and DPV technique, was obtained using 1-naphthyl phosphate as substrate for Ab2–AP after 2 min of incubation. Each point is the average of two determinations.](/image/article/2004/AN/b308052h/b308052h-f2.gif) | ||
| Fig. 2 Titration curves: (a) different dilutions of erythromycin–BSA conjugate [1:50 (●), 1:100 (Δ) and 1:200 (◆)] with different dilutions of anti-erythromycin MAb. (b) different dilutions of tylosin–BSA conjugate [1:10 (Δ), 1:20 (●) and 1:50 (◆)] with different dilutions of anti-tylosin MAb. The current response, detected with screen printed electrodes and DPV technique, was obtained using 1-naphthyl phosphate as substrate for Ab2–AP after 2 min of incubation. Each point is the average of two determinations. | ||
The dilutions chosen above for MAb and conjugate were used to prepare the SPEs for further competitive assays. The standard curves for erythromycin and tylosin generated using these optimised conditions are shown in Fig. 3.
 | ||
| Fig. 3 Standard curves in PBS for erythromycin and tylosin using the optimised ELISA parameters: (●) erythromycin–BSA conjugate dilution 1:50, anti-erythromycin MAb dilution (on the electrode surface) 1:200, (○) tylosin–BSA conjugate dilution 1:20, anti-tylosin Mab dilution (on the electrode surface) 1:50. Each point is the average of two determinations, except of the zero point where n = 6. | ||
The experimental data were fit using a “non-linear four-parameter logistic calibration plot”. The four-parameter logistic18 is given by the equation:
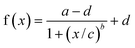 | (1) |
The detection limit (LOD), defined as the concentration corresponding to the f(x) value obtained by subtracting three standard deviations of zero point from the mean of the zero standard measurement (mean value − 3sd),19,20 were 0.2 and 2.0 ng mL−1 for erythromycin and tylosin respectively. Sensitivity, calculated as the amount of macrolide needed to produce a 25% decrease in the signal, was 1.0 ng mL−1 for erythromycin and 3.0 ng mL−1 for tylosin. For tylosin, low detection limit and high sensitivity could only be obtained using a two step competition procedure as reported in the Experimental section.
Measurement of macrolides in bovine muscle
To evaluate the matrix effect on the antigen–antibody interaction, binding curves were carried out by preparing different dilutions of MAb in a blank of bovine muscle extract obtained as for the scheme in Fig. 1. These curves together with the corresponding curves in PBS are reported in Fig. 4 for comparison. As shown, there was only a slight matrix effect for erythromycin, while it was significant in the case of tylosin. Antibody dilutions of 1:200 and 1:30 were chosen to perform the competition of erythromycin and tylosin respectively. | ||
| Fig. 4 Titration curves in (●) PBS and (Δ) in a blank of bovine muscle extract, for: (a) erythromycin–BSA conjugate (1:50) with different dilutions of anti-erythromycin MAb, (b) tylosin–BSA conjugate (1:20) with different dilutions of anti-tylosin MAb. Each point is the average of two determinations. | ||
Calibration curves, obtained using blank meat samples spiked, after extraction, with known amounts of macrolides, are reported in Fig. 5.
 | ||
| Fig. 5 Calibration curves in a blank of bovine muscle extract for erythromycin and tylosin using the optimised ELISA paramters:(▲) erythromycin–BSA conjugate dilution 1:50, anti-erythromycin MAb dilution (on the electrode surface) 1:200, and (Δ) tylosin–BSA conjugate dilution 1:20, anti-tylosin MAb dilution (on the electrode surface) 1:30. Each point is the average of two readings, except of the zero point where n = 6. | ||
The detection limits were 0.3 and 3.0 ng mL−1 for erythromycin and tylosin respectively. Taking into consideration the sample extraction procedure, these limits correspond to 30 µg kg−1 for erythromycin and 30 µg kg−1 for tylosin, that is about 6 and 3 times lower than the MRLs for the two analytes, respectively. Sensitivity was 0.8 ng mL−1 for erythromycin and 12 ng mL−1 for tylosin.
In order to calculate the extraction efficiency of the procedure reported in Fig. 1, aliquots of 2 g of homogenised bovine tissues, spiked with known amounts of erythromycin (ranging from 100–2000 µg kg−1) and tylosin (ranging from 50–2000 µg kg−1), were extracted and analysed. For each concentration level, four different samples were independently processed and analysed using 8 different SPEs. On the basis of the calibration curves prepared in bovine muscle extract it was possible to calculate the extraction efficiency of the two analytes (96–100 ± 10% for both analytes).
To assess the repeatability and accuracy of the immunosensors, two replicates of tissue blank samples spiked with mixtures of the macrolide antibiotics at concentrations of 0.5 × MRL, MRL, 2 × MRL were prepared and analysed on each of four different days for each concentration (n = 16 for each level).
Precision was determined by calculating the RSD% for the replicate measurements and accuracy was calculated by assessing the agreement between measured and nominal concentrations of the spiked samples (RE%). These values are reported in Table (1).
| Analyte | Spiked level/µg kg−1 | Measured concentration (mean ± sd)/µg kg−1 | RSD (%) | RE (%) |
|---|---|---|---|---|
| a Each value is the mean of 16 measurements (8 replicates of spiked samples, each replicate was analysed twice using two different electrodes). | ||||
| Erythromycin | 100 | 106 ± 10 | 9 | 6 |
| 200 | 195 ± 16 | 8 | −2 | |
| 400 | 357 ± 14 | 4 | −11 | |
| Tylosin | 50 | 48 ± 7 | 15 | −4 |
| 100 | 109 ± 9 | 8 | 9 | |
| 200 | 224 ± 29 | 13 | 12 | |
Finally, ten real samples, collected as part of the national program for veterinary drug residue control in Italy, were analysed using the SPE electrochemical ELISA. The reliability of the immunoassay for the determination of the analytes in real samples was demonstrated by comparison of the data with the fully validated confirmatory LC-MS-MS results (Table 2).
| Sample | ELISA | Micro-LC-MS-MS | ELISA/micro-LC-MS-MS (RE%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Erythromycin (mean ± sd)/µg kg−1 | RSD (%) | Tylosin (mean ± sd)/µg kg−1 | RSD (%) | Erythromycin (mean ± sd)/µg kg−1 | RSD (%) | Tylosin (mean ± sd)/µg kg−1 | RSD (%) | Erythromycin | Tylosin | |
| a nd = Not detected. | ||||||||||
| 1 | nda | — | 48 ± 7 | 15 | nd | — | 56 ± 7 | 12 | — | −14 |
| 2 | nd | — | 81 ± 9 | 11 | nd | — | 76 ± 7 | 9 | — | 7 |
| 3 | nd | — | 200 ± 10 | 5 | nd | — | 213 ± 9 | 4 | — | −6 |
| 4 | nd | — | nd | — | nd | — | nd | — | — | — |
| 5 | nd | — | nd | — | nd | — | nd | — | — | — |
| 6 | 360 ± 14 | 4 | nd | — | 382 ± 10 | 3 | nd | — | −6 | — |
| 7 | 264 ± 17 | 6 | nd | — | 245 ± 7 | 3 | nd | — | 8 | — |
| 8 | nd | — | nd | — | nd | — | nd | — | — | — |
| 9 | 199 ± 13 | 7 | nd | — | 183 ± 7 | 4 | nd | — | 9 | — |
| 10 | 118 ± 12 | 10 | nd | — | 108 ± 9 | 8 | nd | — | 9 | — |
Conclusions
Sensitive SPE electrochemical immunosensors, based on competitive ELISA, have been developed for the determination of erythromycin and tylosin in bovine muscle.The disposable immunosensors offer several advantages relative to other reported methods: they are simpler, less time-consuming, inexpensive, sensitive and appear to be extremely useful for screening tests in which it is necessary to monitor erythromycin and tylosin in a large number of samples.
Moreover, while the original Delépine13 procedure used chloroform (designated as a cancer hazard and liver toxin) as solvent for the extraction of the macrolide antibiotics from muscle, our procedure employs only 10 mL of 20% methanol in PBS for each sample. Therefore this extraction procedure can be considered environmentally friendly. Moreover it is faster and simpler since it does not require evaporation of the solvent after extraction, as was the case in our previous work15 based on competitive ELISA coupled with the Delépine extraction procedure.
Acknowledgements
The authors wish to thank the projects ISS 1% e.f. 1999 for financial support. Also Nagwa H. S. Ammida thanks the Secretary of Higher Education, Libya for providing financial support to pursue postgraduate studies in Rome, Italy.References
- Dictionary of Drugs, ed. J. Elks and C. R. Ganelin, Chapman and Hall, London, 1991 Search PubMed.
- S. Omura, Macrolide Antibiotics: Chemistry, Biology and Practice, Academic Press, Orlando, FL, 1984, 26 Search PubMed.
- H. T. Trinh, S. J. Billington, A. C. Field, J. G. Songer and B. H. Jost, Vet. Microbiol., 2002, 85, 353 CrossRef CAS.
- W. A. Moats and M. B. Medina, ACS Symp Ser., 1996, 636, 5.
- Commission of the European Communities, Council Directive EC/96/23, Off. J. Eur. Communities: Legis., 1996, vol. L125, p. 3 Search PubMed.
- J. A. Bernabeu, M. A. Camacho, M. E. Gil-Alegre, V. Ruz and A. I. Torres-Suarez, J. Pharm. Biomed. Anal., 1999, 21, 347 CrossRef CAS.
- Official Methods of Analysis of AOAC International, AOAC International, Arlington, VA, 16th edn., 1995 Search PubMed.
- M. G. Lauridsen, J. Assoc. Off. Anal. Chem., 1988, 71, 921 Search PubMed.
- Y. Tanaka, K. Kimura, Y. Komagata, H. Tsuzuki, H. Comoda and S. Omura, J. Antibiot., 1988, 41, 258 CAS.
- I. Kanfer, M. F. Skinner and R. B. Walker, J. Chromatogr., A, 1998, 812, 255 CrossRef CAS.
- K. Takatsuki, I. Ushizawa and T. Shoji, J. Chromatogr., 1987, 391, 207 CrossRef CAS.
- R. Draisci, L. Palleschi, E. Ferretti, L. Achene and A. Cecilia, J. Chromatogr., A, 2001, 926, 97 CrossRef CAS.
- B. Delépine, D. Hurtaud-Pessel and P. Sanders, J. AOAC Int., 1996, 79, 397 CAS.
- B. Delépine, D. Hurtaud-Pessel and P. Sanders, Analyst, 1994, 119, 2717 RSC.
- R. Draisci, F. delli Quandri, L. Achene, G. Volpe, L. Palleschi and G. Palleschi, Analyst, 2001, 126, 1942 RSC.
- A. Cagnini, I. Palchetti, I. Lionti, M. Mascini and A. P. F. Turner, Sens. Actuators, B, 1995, 24, 85 CrossRef.
- M. Del Carlo, I. Lionti, M. Taccini, A. Cagnini and M. Mascini, Anal. Chim. Acta, 1997, 342, 189 CrossRef CAS.
- T. L. Fare, R. G. Sandberg and D. P. Herzoge, in Environmental Immunochemical Methods: Perspectives and Applications, ed., J. H. Vanehmon and G. L. Gerlach, American Chemical Society, Washington, DC, 1996, p. 240 Search PubMed.
- P. A. Elder, K. H. J. Yeo, J. G. Lewis and J. K. Clifford, Clin. Chim. Acta, 1987, 162, 199 CrossRef CAS.
- F. Nita, R. Schulz and H. H. D. Meyer, Am. J. Vet. Res., 1992, 53, 2213 Search PubMed.
| This journal is © The Royal Society of Chemistry 2004 |
