Dependence of selective enclathration on types of cholic acid crystals
Nungruethai
Yoswathananont
a,
Kazuki
Sada†
a,
Mikiji
Miyata
*a,
Shigendo
Akita
b and
Kazunori
Nakano
b
aDepartment of Material and Life Science, Graduate School of Engineering, Osaka University and Handai FRC, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan. E-mail: miyata@molrec.mls.eng.osaka-u.ac.jp
bNagoya Municipal Industrial Research Institute, 3-4-41 Rokuban, Atsuta-ku, Nagoya 456-0058, Japan. E-mail: nakano.kazunori@nmiri.city.nagoya.jp
First published on 29th November 2002
Abstract
Competitive recrystallizations of cholic acid (CA) from 1 ∶ 1 binary mixtures of seven mono-substituted benzenes are demonstrated. The order of preference for guests to be incorporated into the cholic acid crystals are as follows: benzene, toluene > n-amylbenzene, n-hexylbenzene > ethylbenzene, n-propylbenzene, n-butylbenzene. These seven compounds afford bilayer type inclusion crystals that are classified into four types based on the host frameworks and host–guest stoichiometries. The order of selective enclathration corresponds to the four types as follows: 1 ∶ 1 αG > 2 ∶ 1 αG > 1 ∶ 1 βT or 2 ∶ 1 αT. The preference for the αG type was also confirmed by investigating the host frameworks of the crystals obtained from binary mixtures. The dependence of the selectivity on the different types of CA crystals can be understood in terms of the fit of the guest molecule in the host cavity.
Introduction
There has been growing interest in separation engineering using a crystallization process due to its high efficiency, cost performance, energy saving, less wasted products, and simple procedure.1 In particular, this process has the advantage of being able to separate organic chemicals that are heat sensitive and that decompose at the temperatures required for distillation. However, target compounds are required to form crystalline mass by themselves. This has restricted the use of crystallization as a separation process for organic compounds. Recently, this difficulty has been partly solved by the use of inclusion crystals that are crystalline molecular complexes. In the inclusion compounds, host compounds form open frameworks with void space and the liquid guest compounds are included in the host cavities. For instance, urea is known to form urea adducts with appropriate solutes, and this urea adduction method has been employed in separating n-paraffins. Recently, Hassan and coworkers studied the adsorption of n-paraffins on solid urea rather than into urea crystals to improve the separation.2 As another intriguing example, Toda and coworkers demonstrated the ability of separation and optical resolution of many hosts, including brucine, sparteine, bis-β-naphthol, tartarate derivatives, acetylenic alcohols, and alkylammonium halide.3 Ward and coworkers reported the separation of aromatic compounds using molecular sandwiches based on guanidinium disulfonates.4 However, it is still difficult to design host–guest compounds that display effective separation of guest mixtures. More recently, much attention has been focussed on the rationalization of selective enclathrations on the basis of crystal structures and isomerism of open host frameworks.4Cholic acid (CA), one of the bile acids, has been found to form inclusion crystals with various organic compounds.5 The crystal structures and guest versatility have been investigated, and the separation of mixtures of aniline and nitrobenzene by CA has also been reported.6 More recently, our systematic investigations of CA inclusion crystals with mono-substituted benzenes have revealed that CA forms four different host frameworks depending on the size and shape of the aromatic guest compounds.7 This motivates us to investigate the relationship between the host framework types and the selectivity among mono-substituted benzenes (1–7) that have enough molecular size to afford stable inclusion compounds. In this report, we demonstrate the competitive recrystallizations of CA from the aromatic compounds and reveal what factors dominate the selectivity in such a system using X-ray crystallography.
Experimental
Procedure for competitive recrystallization
All chemicals and solvents were of the commercially available purest grades and used without purification. A host solution was prepared by dissolving CA (130 mg) in butan-1-ol (0.4 ml), while prescribed amounts (1 mmol) of two guest compounds were mixed to make a guest solution. After mixing both solutions in a 13 ml vial, the resulting feed solution was allowed to settle overnight at 20 °C to attain crystallization equilibrium. Inclusion crystals thus obtained, in which the solvent (butan-1-ol) was confirmed not to be included, were filtered out and allowed to settle for some time to remove the adhering solvent and the guests on the crystal surface. The amount of the guests incorporated within the crystal were determined by gas chromatography (HP 5890SeriesII) using a MS detector (HP 5971Series) after dissolving the crystal in methanol.Determination of a single-crystal structure by X-ray crystallography
X-Ray diffractions were collected by Rigaku RAPID imaging plate two-dimensional area detector using graphite-monochromatized Cu-Kα radiation (λ = 1.54178 Å). All the crystallographic calculations were performed by using the TEXSAN software package of the Molecular Structure Corporation.8 Each crystal structure was solved by direct methods (SIR-92) and refined by the full-matrix least squares method. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms attached to carbon atoms were located in the calculated positions.Crystal data for CA–n-butylbenzene (n-butylbenzene was disordered): C24H40O5, M = 408.58, monoclinic, a = 12.782(1), b = 7.9025(6), c = 14.124(2) Å, β = 105.474(5)°, U = 1375.0(2) Å3, T = 213 K, space group P21 (no. 4), Z = 2, 14304 reflections measured, 4656 unique (Rint = 0.051) which were used in all calculations. The final wR(F2) was 0.276.
Crystal data for CA–n-amylbenzene: (C24H40O5)2 + C11H16, M = 965.40, monoclinic, a = 14.1106(9), b = 7.8793(5), c = 25.131(2) Å, β = 96.871(5)°, U = 2774.0(3) Å3, T = 296 K, space group P21 (no. 4), Z = 2, 4998 reflections measured, which were used in all calculations. The final wR(F2) was 0.205.
Crystal data for CA–n-hexylbenzene: (C24H40O5)2 + C12H18, M = 979.43, monoclinic, a = 14.074(1), b = 7.9158(6), c = 25.126(2) Å, β = 96.749(3)°, U = 2779.8(4) Å3, T = 203 K, space group P21 (no. 4), Z = 2, 5334 reflections measured, which were used in all calculations. The final wR(F2) was 0.221.
Determination of crystal structures by powder X-ray diffraction
The host framework types of the inclusion crystals obtained from binary mixtures were determined by X-ray powder diffraction (Rigaku RINT) at room temperature. Diffraction patterns of the 2θ (°) angle, with relative intensity in parentheses, are as follows.Entry 1 (1 + 2): 6.46 (13), 7.04 (100), 7.50 (72), 12.54 (24), 12.88 (20), 13.24 (26), 15.10 (85)
Entry 2 (1 + 3): 6.84 (54), 7.02 (100), 7.52 (46), 12.58 (15), 13.04 (21), 13.30 (27), 15.14 (90)
Entry 3 (1 + 4): 6.74 (13), 7.02 (100), 7.24 (19), 7.44 (63), 12.44 (9), 12.94 (11), 13.26 (18), 14.52 (11), 15.12 (98)
Entry 4 (1 + 5): 7.12 (55), 7.62 (43), 12.58 (11), 13.02 (10), 13.42 (12), 15.26 (100)
Entry 5 (1 + 6): 6.92 (31), 7.44 (75), 12.46 (11), 12.84 (8), 13.22 (22), 15.08 (100)
Entry 6 (1 + 7): 6.96 (10), 7.52 (53), 12.56 (8), 12.94 (5), 13.32 (10), 15.14 (100)
Entry 7 (2 + 3): 6.92 (52), 7.48 (60), 11.04 (10), 12.38 (23), 12.58 (26), 12.92 (42), 13.24 (69), 15.06 (100), 15.34 (57)
Entry 8 (2 + 4): 6.90 (13), 7.46 (47), 11.02 (8), 12.34 (15), 12.52 (19), 12.86 (26), 13.14 (31), 13.44 (29), 15.06 (100), 15.30 (49)
Entry 9 (2 + 5): 6.98 (33), 7.50 (46), 12.68 (6), 12.88 (8), 13.28 (6), 15.12 (100)
Entry 10 (2 + 6): 6.82 (14), 6.98 (19), 7.56 (29), 12.68 (16), 12.98 (14), 13.32 (17), 15.14 (100)
Entry 11 (2 + 7): 6.86 (27), 7.42 (32), 12.42 (12), 12.54 (13), 12.84 (13), 13.18 (21), 15.04 (100)
Entry 12 (3 + 4): 5.80 (16), 7.72 (100), 10.96 (9), 11.16 (14), 12.92 (8), 13.60 (39), 14.42 (22), 15.44 (94), 15.84 (33)
Entry 13 (3 + 5): 6.50 (7), 7.00 (30), 8.22 (30), 12.66 (12), 12.92 (62), 13.20 (13), 13.84 (55), 16.52 (100)
Entry 14 (3 + 6): 7.30 (31), 10.26 (81), 11.74 (45), 12.78 (30), 14.84 (100), 15.66 (22)
Entry 15 (3 + 7): 6.62 (10), 6.90 (39), 7.42 (47), 10.24 (13), 11.64 (11), 12.42 (18), 12.74 (21), 13.06 (18), 15.02 (100)
Entry 16 (4 + 5): 6.98 (18), 7.70 (3), 7.92 (4), 8.16 (31), 10.82 (4), 12.92 (45), 13.76 (13), 15.96 (3), 16.44 (100)
Entry 17 (4 + 6): 6.94 (58), 7.48 (33), 12.52 (18), 12.86 (12), 13.14 (15), 13.44 (34), 15.12 (100)
Entry 18 (4 + 7): 6.92 (33), 7.50 (27), 12.60 (9), 12.80 (6), 13.32 (6), 15.10 (100)
Entry 19 (5 + 6): 7.10 (100), 7.62 (27), 12.66 (21), 12.98 (13), 13.28 (10), 13.56 (30), 15.24 (87)
Entry 20 (5 + 7): 6.92 (100), 7.46 (25), 12.50 (16), 12.84 (7), 13.10 (6), 13.38 (13), 15.08 (57)
Entry 21 (6 + 7): 7.08 (56), 7.64 (38), 12.62 (12), 13.00 (12), 13.50 (12), 15.22 (100)
Molecular graphics and calculations
Cross-sections of host channels were depicted by using MODRASTE.9 The atomic radii of hydrogen and carbon in the cross-sectional views are 1.20 Å and 1.60 Å, respectively.PCcavity was calculated from the volumes of the host cavity and the guest molecule.7 The volumes of the host cavities were calculated from the atomic coordinations by using the Free Volume program in the Cerius2 (version 4.0) software package.10 The atomic radii were determined to have the following values by this method: hydrogen = 1.20 Å, carbon = 1.70 Å, and oxygen = 1.60 Å.
Results and discussion
Competitive recrystallizations
Inclusion compounds of CA with mono-substituted benzenes were obtained easily using butan-1-ol as the solvent.7 The experiments described here were carried out using guest-rich mixtures, i.e. crystallization was attained from solutions containing excess amounts of the two guests. The separation factor (SF) is defined and simplified under the present conditions in which the molar concentration of each guest was the same or much higher than that of the host to| SF = ([A]cry/[A]sol)/([B]cry/[B]sol) = [A]cry/[B]cry |
Competitive recrystallization was carried out using equimolar mixtures of n-alkylbenzenes from benzene (1) to n-hexylbenzene (7), as shown in Table 1. The separation factors for all the binary systems of guest 1 and a series of n-alkylbenzenes (2–7) were more than unity, indicating that guest 1 is preferentially incorporated into the CA crystals. The selectivity increased with an increase in the number of methylene groups in the guest molecules and the separation factor reached as high as 7.6 for 5, and then further increase in the length of the methylene chain caused a lowering of the separation factor. A similar trend can be seen for the mixtures of 2, and the highest separation factor, 15.1, was obtained from a mixture of 2 and 4. Moreover, guests 6 and 7 are favorably included in CA compared to the other three (3, 4, and 5). The following combinations gave no or less selective enclathrations (0.75 < SF < 1.5); 1
+
2, 3
+
4, 3
+
5, and 6
+
7
(entries 1, 12, 13, and 21). From the results, the order of preference for inclusion in CA is as follows:
| 1, 2>6, 7>3, 4, 5 |
| Entry | Guest A (Host framework, host ∶ guest ratio)a | Guest B (Host framework, host ∶ guest ratio)a | [A]cry/[A]cry + [B]cry (%) | [B]cry/[A]cry + [B]cry (%) | SF | Host framework of a 1 ∶ 1 mixture |
|---|---|---|---|---|---|---|
| a Host framework and host ∶ guest ratio refer to those of crystals obtained from each pure guest. | ||||||
| 1 | 1 (αG, 1 ∶ 1) | 2 (αG, 1 ∶ 1) | 54 | 46 | 1.2 | αG |
| 2 | 1 (αG, 1 ∶ 1) | 3 (βT, 1 ∶ 1) | 81 | 19 | 4.3 | α G |
| 3 | 1 (αG, 1 ∶ 1) | 4 (βT, 1 ∶ 1) | 84 | 16 | 5.3 | α G + βT |
| 4 | 1 (αG, 1 ∶ 1) | 5 (αT, 2 ∶ 1) | 88 | 12 | 7.6 | α G |
| 5 | 1 (αG, 1 ∶ 1) | 6 (αG, 2 ∶ 1) | 80 | 20 | 4.0 | α G |
| 6 | 1 (αG, 1 ∶ 1) | 7 (αG, 2 ∶ 1) | 72 | 28 | 2.6 | α G |
| 7 | 2 (αG, 1 ∶ 1) | 3 (βT, 1 ∶ 1) | 81 | 19 | 4.4 | α G |
| 8 | 2 (αG, 1 ∶ 1) | 4 (βT, 1 ∶ 1) | 94 | 6 | 15.1 | α G |
| 9 | 2 (αG, 1 ∶ 1) | 5 (αT, 2 ∶ 1) | 91 | 9 | 9.6 | α G |
| 10 | 2 (αG, 1 ∶ 1) | 6 (αG, 2 ∶ 1) | 82 | 18 | 4.7 | α G |
| 11 | 2 (αG, 1 ∶ 1) | 7 (αG, 2 ∶ 1) | 78 | 22 | 3.5 | α G |
| 12 | 3 (βT, 1 ∶ 1) | 4 (βT, 1 ∶ 1) | 59 | 41 | 1.4 | β T |
| 13 | 3 (βT, 1 ∶ 1) | 5 (αT, 2 ∶ 1) | 48 | 52 | 0.91 | α T |
| 14 | 3 (βT, 1 ∶ 1) | 6 (αG, 2 ∶ 1) | 32 | 68 | 0.48 | α G |
| 15 | 3 (βT, 1 ∶ 1) | 7 (αG, 2 ∶ 1) | 43 | 57 | 0.75 | α G |
| 16 | 4 (βT, 1 ∶ 1) | 5 (αT, 2 ∶ 1) | 30 | 70 | 0.43 | α T |
| 17 | 4 (βT, 1 ∶ 1) | 6 (αG, 2 ∶ 1) | 13 | 87 | 0.15 | α G |
| 18 | 4 (βT, 1 ∶ 1) | 7 (αG, 2 ∶ 1) | 14 | 86 | 0.16 | α G |
| 19 | 5 (αT, 2 ∶ 1) | 6 (αG, 2 ∶ 1) | 26 | 74 | 0.35 | α G + αT |
| 20 | 5 (αT, 2 ∶ 1) | 7 (αG, 2 ∶ 1) | 33 | 67 | 0.50 | α G |
| 21 | 6 (αG, 2 ∶ 1) | 7 (αG, 2 ∶ 1) | 55 | 45 | 1.1 | α G |
Crystal structures of CA clathrates with n-alkylbenzenes
In order to clarify the guest selectivities in the competitive recrystallizations, we investigated the crystal structures of CA clathrates including the pure guest compounds, as shown in Fig. 1. Table 2 summarizes the lattice parameters, the types of the host frameworks, the host–guest molar ratios, and the PCcavity. They all have bilayer structures composed of hydrophilic and lipophilic layers and the guest molecules are incorporated into the one-dimensional cavity in the lipophilic layer. The structures are classified into three types of host frameworks, αG, αT, and βT, based on the difference in the interdigitation behaviour of the methyl groups at the lipophilic faces (α and β types) and in the steroidal side chain conformations (G (gauche) and T (trans) types).5 The guests 1, 2, 6, and 7 are included in the αG type. The others, 3 and 4, are of the βT type. However, in the case of guest 5, the orientation of the guest compounds in the host cavity could not be confirmed because of the disorder in the phenyl ring, while the host framework is found to be of the αT type. Small guest molecules (1–4) are included at 1 ∶ 1 host–guest ratios, and guests with more than nine carbon atoms (5–7) are incorporated in a 2 ∶ 1 ratio. Namely, CA inclusion crystals with seven mono-substituted benzenes (1–7) are classified into the four types, 1 ∶ 1 αG, 1 ∶ 1 βT, 2 ∶ 1 αG, and 2 ∶ 1 αT, based on the host framework and host–guest ratio. In addition, we calculated the PCcavity, the volume ratio of the guest molecules to the host cavities, to estimate the size complementarity in each crystal. These values were in the range of 52–70%, indicating that all the aromatic guests have a sufficiently large molecular size to afford stable inclusion compounds.7| Guest | Space group | a/Å | b/Å | c/Å | β/° | V/Å3 | Host framework | Host ∶ guest ratio | PCcavity (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P21 | 13.63 | 8.04 | 14.08 | 114.3 | 1406 | α G | 1 ∶ 1 | 56 | 7 |
| 2 | P21 | 13.74 | 8.04 | 14.01 | 114.1 | 1421 | α G | 1 ∶ 1 | 60 | 7 |
| 3 | P21 | 12.41 | 7.83 | 16.28 | 111.8 | 1469 | β T | 1 ∶ 1 | 61 | 7 |
| 4 | P21 | 12.07 | 7.84 | 16.25 | 109.8 | 1447 | β T | 1 ∶ 1 | 70 | 7 |
| 5 | P21 | 12.78 | 7.90 | 14.12 | 105.5 | 1375 | α T | 2 ∶ 1 | 52 | This work |
| 6 | P21 | 14.11 | 7.87 | 25.13 | 96.8 | 2774 | α G | 2 ∶ 1 | 54 | This work |
| 7 | P21 | 14.07 | 7.91 | 25.12 | 96.7 | 2779 | α G | 2 ∶ 1 | 60 | This work |
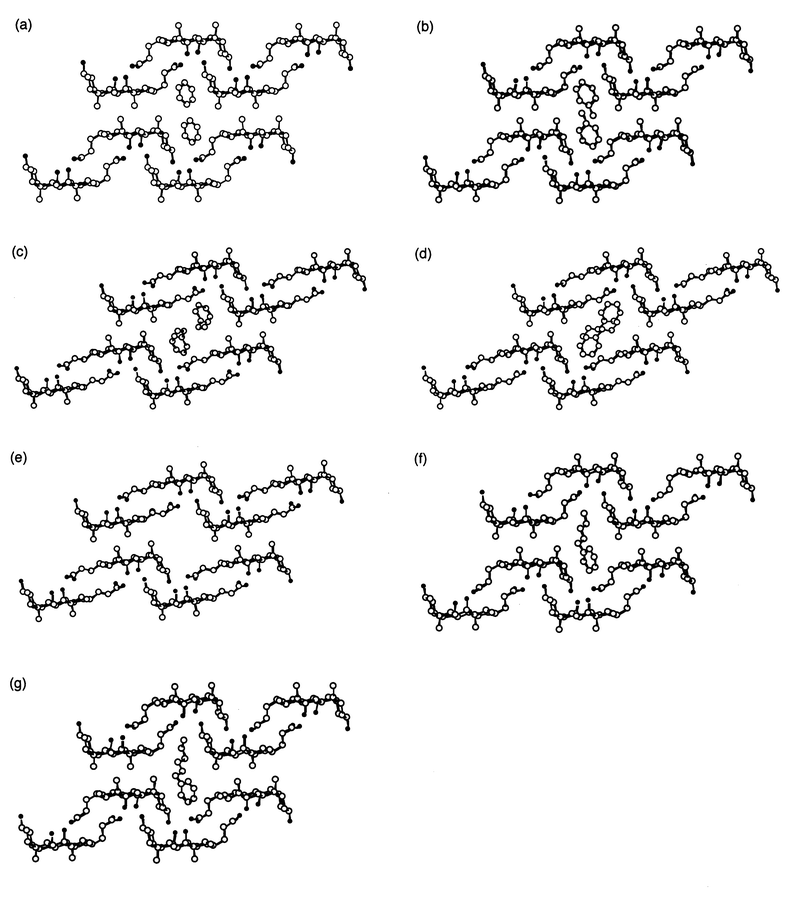 | ||
| Fig. 1 Crystal structures of CA with (a) 1, (b) 2, (c) 3, (d) 4, (e) 5, (f) 6, and (g) 7, respectively. The figures are viewed down along the crystallographic b-axis. Hydrogen atoms are omitted for clarity. Carbon and oxygen atoms are represented by open and filled circles, respectively. | ||
Elucidation of selectivities
The selectivities for the guest compounds by the enclathrations seem to be dependent on the four types of frameworks mentioned above. The preference order is reviewed as below:| 1 ∶ 1 αG > 2 ∶ 1 αG > 1 ∶ 1 βT or 2 ∶ 1 αT |
This order indicates that the αG type host framework is more favorable than the other two trans-type (βT or αT) framework, and that the 1 ∶ 1 αG type is more favorable than the 2 ∶ 1 αG. In addition, this order agrees with the fact that less selective enclathrations (entry 1, 12, and 21) were observed when they construct the same host frameworks at the same host-guest ratios in a single system. For example, both 1 and 2 can be included in the same 1 ∶ 1 αG type. In the same way, less selective enclathrations were also observed in the cases of 3vs. 4 (1 ∶ 1 βT) and 6vs. 7 (2 ∶ 1 αG).
Next, the host frameworks of the crystals obtained from competitive experiments were determined by powder X-ray diffraction, as shown in Table 1. All the resulting crystals have either one of the host frameworks (αG, αT, and βT) or a combination of these. When both of the guest compounds are included in the same host framework (entries 1, 5, 6, 10–12, and 21), the competitive recrystallizations provide the same host frameworks. On the other hand, when they are included in different host frameworks, two types of inclusion crystals result; one is a homogenous crystal with the mixed guests in the host cavity (entries 2, 4, 7–9, 13–18, and 20) and the other is a mixture of two different inclusion crystals (entries 3 and 19). In the former, the host frameworks are the same as those of the predominant guests, and in the latter the guests are incorporated into each host framework as if they were in the pure state. When one of the guest compounds in the guest mixtures gives the αG host framework in the pure state (entries 2, 4, 7–9, 14, 15, 17, 18, and 20), the αG host framework forms exclusively and both the guests are incorporated into the host cavity. This suggests that the αG type is preferred to αT and βT. In the other two cases (entries 13 and 16), only the αT type host frameworks are obtained, indicating that αT is more preferable to βT.
In order to clarify the factors that influence the selective inclusions, we compared the PCcavity, which represents the packing efficiency of the guest compounds in the host cavities, that is, size complementarity. The order of PCcavity (4 > 2, 3, 7 > 1, 5, 6) has no correlation with that of the selectivities, indicating that the PCcavity is not a suitable measure for explaining the guest selectivities. It would be due to enough packing of the guest compounds in the host cavities. From the results, the shape complementarity plays an important role. Fig. 2 illustrates typical cross-sections of the host cavities sliced parallel to the axis of the one-dimensional host cavity at a height that shows the cross-sections surrounded by the side chains. The host cavity of an αG type framework has a square groove accommodating the phenyl ring of the guest molecule (Fig. 2 (a), (d)), while those of the trans type frameworks have triangle ones (Fig. 2 (b), (c)). These figures illustrate that the αG types have host cavities that are more appropriate for a phenyl ring than the αT and βT types. From the results, the αG type predominantly forms from guest mixtures. The host–guest ratios also play an important role in the fit of the guest molecule in the host cavity. In the case of the αG type framework, the guest compounds that give inclusion crystals in 1 ∶ 1 ratios are included more efficiently than those in inclusion crystals in 2 ∶ 1 ratios. In the former, all the square grooves of the host cavities along the two-fold screw axis are occupied by the phenyl ring of the guest compounds, but in the latter, half of them are occupied by the alkyl group. The shape complementarity between a groove and the guest molecule causes the selectivity to depend on the four types of CA crystals.
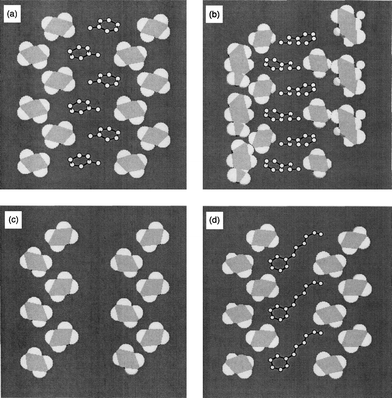 | ||
| Fig. 2 Cross-sections of the host channels sliced parallel to the direction of the channel (carbon and hydrogen atoms are represented by gray and white, respectively) with arrays of included guest molecules (hydrogen atoms are omitted for clarity, and carbon atoms are represented by open circles):(a) 2, (b) 4, (c) 5, and (d) 7. | ||
Conclusions
We have demonstrated the competitive recrystallizations of CA from 1 ∶ 1 binary mixtures of seven mono-substituted benzenes that afford inclusion compounds by recrystallization from pure liquids. The order of the preference to be included in CA crystals is as follows: 1, 2 > 6, 7 > 3, 4, 5, which is attributed to the dominant formation of the αG host framework in 1 ∶ 1 host–guest ratios of the four host frameworks. This can be understood in terms of the suitable fitting of the phenyl ring of the guest molecule to a square groove of the αG host framework. Finally, we want to emphasize here that CA exhibits shape-selective recognition of aromatic guests that are of sufficiently large molecular size to give stable inclusion compounds. Since CA can form inclusion compounds with a variety of organic compounds by forming diverse host frameworks with changing host–guest ratios, CA can be used for molecular recognition of various guest compounds. We will now investigate extensively competitive recrystallizations using CA.Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.References
- C. G. Moyers, Jr., and R. W. Rousseau, Handbook of Separation process Technology, ed. R. W. Rousseau, Wiley, New York, 1987, ch. 11, p. 578 Search PubMed.
- (a) N. M. Hassan, R. S. Al-Ameeri and F. A. Oweysi, Sep. Sci. Technol., 1994, 29, 401 Search PubMed; (b) N. M. Hassan, R. S. Al-Ameeri and F. A. Oweysi, Sep. Sci. Technol., 1994, 29, 897 Search PubMed.
- (a) F. Toda, Advances in Supramolecular Chemistry, ed. G. W. Gokel, JAI Press, London, 1992, Vol. 2, p. 141 Search PubMed; (b) F. Toda, K. Tanaka, H. Miyamoto, H. Koshima, I. Miyahara and K. Hirotsu, J. Chem. Soc., Perkin Trans. 2, 1997, 1877 RSC; (c) M. R. Caira, L. R. Nassimbeni, F. Toda and D. Vujovic, J. Am. Chem. Soc., 2000, 122, 9367 CrossRef CAS; (d) K. Tanaka, K. Tamura and F. Toda, J. Chem. Soc., Perkin Trans. 2, 1995, 1571 RSC.
- A. M. Pivovar, K. T. Holman and M. D. Ward, Chem. Mater., 2001, 13, 3018 CrossRef.
- M. Miyata and K. Sada, Comprehensive Supramolecular Chemistry, Solid-state Supramolecular Chemistry: Crystal Engineering, Ed. D. D. MacNicol, F. Toda and R. Bishop, Pergamon, Oxford, 1996, Vol. 6, p. 147 Search PubMed.
- M. R. Caira, L. R. Nassimbeni and J. L. Scott, J. Chem. Soc., Chem. Commun., 1993, 612 RSC.
- K. Nakano, Y. Kurozumi, K. Sada and M. Miyata, Chem. Eur. J., 2001, 7, 209 CrossRef CAS.
- TEXSAN, X-ray Structure Analysis Package, Molecular Structure Corporation, The Woodlands, TX, 1985.
- H. Nakano, Molecular Graphics, Science House, Tokyo, 1987 Search PubMed.
- Cerius2, Molecular Simulation Software, Molecular Simulations Inc., San Diego, CA , 1998.
Footnote |
| † Present address: Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan |
| This journal is © The Royal Society of Chemistry 2003 |