Detection of a metallo-β-lactamase (IMP-1) by fluorescent probes having dansyl and thiol groups†
Hiromasa
Kurosaki
a,
Hisami
Yasuzawa
a,
Yoshihiro
Yamaguchi
a,
Wanchun
Jin
a,
Yoshichika
Arakawa
b and
Masafumi
Goto
*a
aFaculty of Pharmaceutical Sciences, Kumamoto University, Oe-honmachi 5-1, Kumamoto 862-0973, Japan. E-mail: gotomphi@gpo.kumamoto-u.ac.jp; Fax: +81-96-371-4314; Tel: +81-96-371-4310
bDepartment of Bacterial and Blood Products, National Institute of Infectious Diseases, Gakuen 4-7-1, Musashi-Murayama, Tokyo 208-0011, Japan. E-mail: yarakawa@nih.go.jp; Fax: +81-42-561-7173; Tel: +81-42-561-0771
First published on 8th November 2002
Abstract
A fluorescent probe for the detection of a metallo-β-lactamase (IMP-1), N-[2-(5-dimethylaminonaphthalen-1-ylsulfonylamino)ethyl]-3-mercaptopropionamide (DansylC2SH), 1, was designed based on combining the inhibitory function of mercaptocarboxylate and a fluorophore. The binding of 1 to IMP-1 was investigated by fluorescence spectroscopy. Compound 1 can act as fluorescent probe for detecting IMP-1 selectively.
Introduction
β-Lactam agents, which inhibit the bacterial peptidoglycan synthetic system for cell wall construction, are the antibiotics widely used in medical treatment of infectious diseases.1,2 Bacteria have acquired various means of resistance to these antibiotics. The production of β-lactamase is a major means of this, and the number of the resistant strains of bacteria have increased since the discovery of penicillin.β-lactamases hydrolyse the endocyclic amide bond of β-lactam antibiotics to inactivate them3–5 and have been grouped into four classes:6 Those of classes A, C, and D have a serine residue in the active centre but class B β-lactamases require divalent metal cation(s) such as zinc(II) in the active centre and are called metallo-β-lactamase.
Metallo-β-lactamases have been first discovered in the middle of 1960s from Bacillus cereus.7–9 Since 1990, this class of enzyme is reported to be isolated from carbapenem-resistant clinical isolates such as Bacteroides fragilis,10–12Aeromonas hydrophila ,13Xanthomonas (Stenotrophomonas) maltophilia,14,15 and Serratia marcescens.16,17 The enzymes of this class B have a broad substrate spectrum and hydrolyse most β-lactam antibiotics including carbapenems, such as imipenem and meropenem.18 Moreover, serine β-lactamase inhibitors, sulbactam and clavulanic acid, do not inhibit the metallo-β-lactamase. Recently intense efforts are focused on developing specific inhibitors of the metallo-β-lactamases in combination with β-lactams.19–21
Among metallo-β-lactamases, IMP-1 is transferable. This enzyme was first identified in clinical isolates from Serratia marcescens but now production of IMP-1 is spreading over various bacterial pathogens not only in Japan but also in Europe22 because the gene resides on a mobile gene cassette within integron.23 We have found that mercaptocarboxylates such as 2-mercaptopropionic acid and 3-mercaptopropionic acid are potent inhibitors of IMP-1.24 An X-ray crystal structure of the complex of wild-type IMP-1 with a mercaptocarboxylate inhibitor (see Fig. 1) has revealed that the inhibitor binds to two zinc ions in the active centre.25
![Structure of the mercaptocarboxylate inhibitor, 2-[5-(tetrazol-1-ylmethyl)thien-3-yl]-N-[2-(mercaptomethyl)-4-(phenylbutyrylglycine)].](/image/article/2003/OB/b209086d/b209086d-f1.gif) | ||
| Fig. 1 Structure of the mercaptocarboxylate inhibitor, 2-[5-(tetrazol-1-ylmethyl)thien-3-yl]-N-[2-(mercaptomethyl)-4-(phenylbutyrylglycine)]. | ||
For metallo-β-lactamases, few techniques for detection are so far known: PCR analysis26 and a disc method27 which exploits the inhibitory action of simple mercaptocarboxylates. The former gives generally reliable and satisfactory results for the detection of IMP-1 but is not suitable for practical use in daily applications in clinical laboratories due to the cost and time. On the other hand, the latter is not quantitative although it provides a simple, rapid and inexpensive method for screening IMP-1. To overcome these shortcomings, the use of fluorescence is desirable because it provides a more rapid and quantitative method.
In order to devise a fluorometric method for detecting metallo-β-lactamases, we designed a new fluorescent probe, N-[2-(5-dimethylaminonaphthalen-1-ylsulfonylamino)ethyl]-3-mercaptopropionamide (DansylC2SH), 1, in which the fluorescent group (dansyl) and the inhibitor (3-mercaptopropionic acid) are linked via ethylenediamine as a spacer. Compound 1 is capable of functioning as both a detecting agent and an inhibitor of IMP-1. We describe herein the synthesis and fluorescence properties of 1 with metallo-β-lactamase (IMP-1).
Results and discussion
As shown in Scheme 1, coupling of dansylethylenediamine with an equimolar amount of 3-mercaptopropionic acid using DCC as a condensing agent in ethyl acetate followed by column chromatography on silica gel gave the desired compound 1 as a yellow oil in 77% yield. Characterization and purity (∼99%) of the compound are determined by TLC, NMR spectroscopy and mass spectrometry.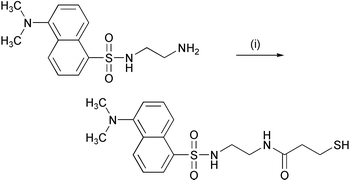 | ||
| Scheme 1 Reagents: (i) DCC, 3-mercaptopropionic acid, and ethyl acetate. | ||
Compound 1 excited at 340 nm (ε = 3870 M−1cm−1) in 50 mM Tris–HCl buffer (pH 7.4) containing 0.5 M NaCl and 10% methanol exhibited an emission maximum at 535 nm (Φ = 0.014). When a 1 µM IMP-1 buffered solution was added to a 1 µM solution of 1, the fluorescence intensity of DansylC2SH increased 2.5-fold and the emission maximum shifted from 535 nm to 525 nm (Fig. 2a).
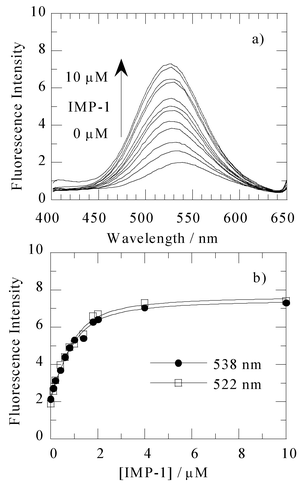 | ||
| Fig. 2 (a) Fluorescence emission spectra (excitation 340 nm) of 1 µM 1 in the presence of various concentrations of the IMP-1 metallo-β-lactamase ranging from 0 to 10 µM. These spectra were measured at 25 °C, in 50 mM Tris–HCl buffered solution (pH 7.4) containing 0.5 M NaCl and 10% methanol. (b) Fluorescence intensity of emission as a function of the added IMP-1 metallo-β-lactamase concentration monitored at 522 and 538 nm. An apparent KD of 0.36 µM was observed by analysing the data with a nonlinear least-squares fitting.28 | ||
The binding of 1 with IMP-1 was examined by a fluorescence spectrophotometric titration in 50 mM Tris–HCl buffer (pH 7.4) containing 0.5 M NaCl and 10% methanol, and the results are shown in Fig. 2b. Titration of a 1 µM solution of 1 with the enzyme, IMP-1, resulted in an increase in the emission intensity with an increasing concentration of IMP-1, gradually reaching saturation above 1 µM of the IMP-1 metallo-β-lactamase (up to 5-fold fluorescence intensity). The apparent dissociation constant, KD, was estimated by a non-linear fitting of the titration data, being 0.36 µM.
Under the same conditions, the fluorescence intensity of 1 did not change upon addition of a 1 µM solution of the apo-enzyme of IMP-1, apo-IMP-1 (Fig. 3), which contained 0.07 mol of Zn(II) ions per 1 mol of the enzyme (determined by atomic absorption spectrometry).
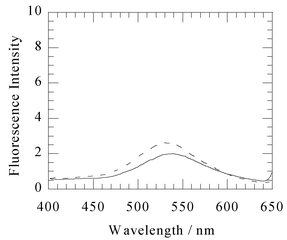 | ||
| Fig. 3 Fluorescence emission spectra (excitation 340 nm) of 1 µM 1 (solid line) and after addition of 1 µM of the apo-enzyme of IMP-1 (dashed line). These spectra were measured at 25 °C, in 50 mM Tris–HCl buffered solution (pH 7.4) containing 0.5 M NaCl and 10% methanol. | ||
Fluorescence enhancement and hypochromic shifts indicate that the thiol group in 1 coordinates to the Zn(II) ion(s) in the active site of the IMP-1 metallo-β-lactamase, and compound 1 fits into the hydrophobic pocket.
It is to be noted that this fluorescence enhancement was not observed with a 1 µM solution of 1 upon addition of 2 µM solutions of albumin or carbonic anhydrase the latter of which has a zinc(II) ion in its active centre.29 In addition, other fluorescent probes with dansyl chromophores: dansylethylenediamine, 2-(dansylamino)ethanol and dansylsulfonamide (DASA)30,31 which are known to form highly fluorescent complexes with carbonic anhydrase and bovine serum albumin, exhibited no fluorescence enhancement with IMP-1. Therefore, the mercapto group on the fluorescent probe may be required for specific binding to the IMP-1 metallo-β-lactamase.
As IMP-1 metallo-β-lactamase has six tryptophan residues, the tryptophan fluorescence with excitation at 280 nm was next examined as a way of improving the specificity of this method. For 280 nm excitation, the emission maximum of a 1 µM solution of IMP-1 in the absence of 1 occurs near at 340 nm. Addition of a 1 µM solution of 1 to a 1 µM solution of IMP-1 resulted in 30% quenching of tryptophan fluorescence and the appearance of a new band at 525 nm (Fig. 4). The latter band is analogous to the spectra observed for 1 with 340 nm excitation. The decrease at 340 nm and the increase at 525 nm of the fluorescence intensity showed concentration dependence on 1. This result can be explained on the basis of a Fluorescence Resonance Energy Transfer (FRET) process32 from the tryptophan residue to the dansyl group in 1; the emission spectrum of the IMP-1 metallo-β-lactamase overlaps with the absorption maximum at 340 nm of 1 with a molar absorptivity of 3870 M−1cm−1. Similar behaviour has also been reported with the protein interactions of fluorescent agents (for instance, bovine carbonic anhydrase–DNSA complex).30
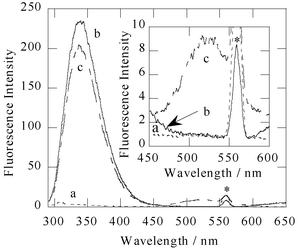 | ||
| Fig. 4 Fluorescence emission spectra (excitation 280 nm) of 1 µM 1 (a), 1 µM IMP-1 (b), and after addition of 1 µM 1 to 1 µM IMP-1 (c). These spectra were measured at 25 °C, in a 50 mM Tris–HCl buffered solution (pH 7.4) containing 0.5 M NaCl and 10% methanol. Inset: the 450–600 nm region, expanded. The peak marked with an asterisk denotes second scattered light of the solvent. | ||
In order to test the effect of 1 on the inhibition of the IMP-1 metallo-β-lactamase, we determined the half-maximal inhibitory concentration, IC50 using nitrocefin as a reporting substrate. The concentration-response curve for the inhibition of IMP-1 by 1 is shown in Fig. 5. Compound 1 was found to have an IC50 of 5.2 µM. Moreover, the inhibition constant, Ki, for 1 according to a competitive inhibition pattern was calculated with fitting of kinetic data, giving a Ki value of 1.0 µM, which is in good agreement with the KD value of 0.36 µM obtained by fluorescence spectrophotometry.
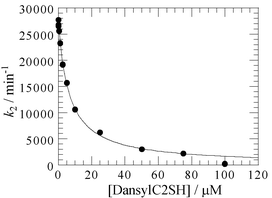 | ||
| Fig. 5 Concentration-response curve for the inhibition of IMP-1 by 1. Ki value is calculated assuming a competitive inhibition. | ||
Concha et al., have reported the X-ray crystal structure of the complex of IMP-1 with a mercaptocarboxylate inhibitor (2-[5-(tetrazol-1-ylmethyl)thien-3-yl]-N-[2-(mercaptomethyl)-4-(phenylbutyrylglycine)], Fig. 1).25 In this structure, the inhibitor binds in the active site; the inhibitor's thiolate bridges the two zinc(II) ions displacing the bridging water, and the phenylbutyl moiety binds in a hydrophobic pocket at the base of the flap. The inhibitor's carboxylate group interacts with Lys161 and the main chain amide nitrogen of Asn167.
The coordination of the inhibitor's thiolate group to a metal ion in the active site was probed by electronic spectroscopy of cobalt(II)-substituted IMP-1 with 3-mercaptopropan-1-ol. The Co(II)-substituted enzyme, Co(II)–IMP-1, was prepared from addition of up to 2 equimolar amounts of CoSO4·7H2O to apo-IMP-1 in 50 mM Tris–HCl (pH 7.4) comprising 1.0 M NaCl and 30% glycerine.
The electronic spectrum of Co(II)–IMP-1 showed intense absorptions at 525, 552, 612 and 639 nm in the visible region, which can be assigned to a d–d transition in the Co site 1. In addition, an intense absorption was observed at 344 nm, being characteristic of a ligand-to-metal charge transfer from the thiolate of the cysteine residue to cobalt(II) in site 2.
When 3-mercaptopropan-1-ol, which acts as a reversible inhibitor for IMP-1,24 was added to Co(II)–IMP-1, the intensity of the absorption band at 344 nm increased and the original bands at 527, 597, 635, and 636 nm shifted to 570, 620, and 689 nm with an increase in intensity. These spectral changes suggest that the thiol group in mercapto derivatives coordinates to the metal ion in the active site.
Conclusion
We have developed new type of fluorescent probe capable of detecting the IMP-1 metallo-β-lactamase selectively and with high sensitivity. It is likely that this fluorescent probe will be applicable as a detecting agent for the metallo-β-lactamases in clinical laboratories.Experimental
Synthesis of N-[2-(5-dimethylaminonaphthalen-1-ylsulfonylamino)ethyl]-3-mercaptopropionamide (DansylC2SH), 1
A solution of dansylethylenediamine (3.94 g, 13.43 mmol) and 3-mercaptopropionic acid (1.42 g 13.4 mmol) was dissolved in 200 mL of ethyl acetate, and stirred at 0 °C under argon. After 30 min, N,N′-dicyclohexylcarbodiimide (DCC, 2.77 g, 13.4 mmol) in 100 mL of ethyl acetate was added to the reaction mixture and the mixture was stirred for additional 38 h at room temperature. The reaction mixture was evaporated under reduced pressure and ethyl acetate (ca. 200 mL) was added to the resulting residue to remove N,N-dicyclohexylurea. After filtration, the filtrate was evaporated to dryness and the residue was purified by column chromatography on silica gel (eluent, 20% methanol–chloroform) to give 1 (3.94 g, 77%) as yellow oil. 1H NMR (CDCl3): δ 1.51 (t, 1H), 2.29 (t, 2H), 2.66 (q, 2H), 2.89 (s, 6H), 3.06 (q, 2H), 3.32 (q, 2H), 5.80 (t, 1H), 6.20 (t, 1H), 7.19 (d, 1H, J = 7.32 Hz), 7.52 (t, 1H, J = 7.32 Hz), 7.57 (t, 1H, J = 7.93 Hz), 8.23 (d, 1H, J = 8.55 Hz), 8.28 (d, 1H, J = 8.54 Hz), 8.55 (d, 1H, J = 8.54 Hz). 13C NMR (CDCl3): δ 20.31, 39.40, 40.08, 43.09, 45.41, 115.37, 118.60, 123.25, 128.63, 129.50, 129.68, 129.98, 130.75, 134.35, 152.18, 171.67. HRFABMS: m/z 381.1213 [calcd for C17H23N3S2 381.1181].Acknowledgements
This work was supported by the Research Foundation for Kumamoto Techno Association and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture (No. 13672257).References
- J. R. Knowles, Acc. Chem. Res., 1985, 18, 97 CrossRef CAS.
- Z. Wang, W. Fast, A. M. Valentine and S. J. Benkovic, Curr. Opin. Chem. Biol., 1999, 3, 614 CrossRef CAS.
- M. I. Page and A. P. Laws, Chem. Commun., 1998, 1609 RSC.
- M. I. Page, Curr. Pharm. Des., 1999, 5, 895 Search PubMed.
- J. A. Cricco and A. J. Vila, Curr. Pharm. Des., 1999, 5, 915 Search PubMed.
- R. P. Ambler, Philos. Trans. R. Soc. London, Ser. B., 1980, 289, 321 Search PubMed.
- L. D. Sabath and E. P. Abraham, Biochem. J., 1966, 98, 11c CAS.
- S. Kuwabara and E. P. Abraham, Biochem. J., 1967, 103, 27c CAS.
- R. B. Davies and E. P. Abraham, Biochem. J., 1974, 143, 115 CAS.
- G. J. Cuchural, Jr., M. H. Malamy and F. P. Tally, Antimicrob. Agents Chemother., 1986, 30, 645 CAS.
- B. A. Rasmussen, Y. Gluzman and F. P. Tally, Antimicrob. Agents Chemother., 1990, 34, 1590 CAS.
- K. Bandoh, Y. Muto, K. Watanabe, N. Katoh and K. Ueno, Antimicrob. Agents Chemother., 1991, 35, 371 CAS.
- O. Massidda, G. M. Rossolini and G. Satta, J. Bacteriol., 1991, 173, 4611 CAS.
- T. R. Walsh, L. Hall, S. J. Assinder, W. W. Nichols, S. J. Cartwright, A. P. MacGowan and P. M. Bennett, Biochim. Biophys. Acta, 1994, 1218, 199 CAS.
- M. W. Crowder, T. R. Walsh, L. Banovic, M. Pettit and J. Spencer, Antimicrob. Agents Chemother., 1998, 42, 921 CAS.
- E. Osano, Y. Arakawa, R. Wacharotayankun, M. Ohta, T. Horii, H. Ito, F. Yoshimura and N. Kato, Antimicrob. Agents Chemother., 1994, 38, 71 CAS.
- H. Ito, Y. Arakawa, S. Ohsuka, R. Wacharotayankun, N. Kato and M. Ohta, Antimicrob. Agents Chemother., 1995, 39, 824 CAS.
- D. M. Livermore and N. Woodford, Curr. Opin. Microbiol., 2000, 3, 489 CrossRef CAS.
- D. J. Payne, W. Du and J. H. Bateson, Expert Opin. Invest. Drugs, 2000, 9, 247 Search PubMed.
- J. H. Toney, G. G. Hammond, P. M. D. Fitzgerald, N. Sharma, J. M. Balkovec, G. P. Rouen, S. H. Olson, M. L. Hammond, M. L. Greenlee and Y.-D. Gao, J. Biol. Chem., 2001, 276, 31913 CrossRef CAS.
- D. J. Payne, J. A. Hueso-Rodríguez, H. Boyd, N. O. Concha, C. A. Janson, M. Gilpin, J. H. Bateson, C. Cheeven, N. L. Niconovich, S. Pearson, S. Rittenhouse, D. Tew, E. Díez, P. Pérez, J. de la Fuente, M. Rees and A. Rivera-Sagredo, Antimicrob. Agents Chemother., 2002, 46, 1880 CrossRef CAS.
- G. Cornaglia, M. L. Riccio, A. Mazzariol, L. Lauretti, R. Fontana and G. M. Rossolini, Lancet, 1999, 353, 899 CrossRef CAS.
- Y. Arakawa, M. Murakami, K. Suizuki, H. Ito, R. Wacharotayankun, S. Ohsuka, N. Kato and M. Ohta, Antimicrob. Agents Chemother., 1995, 39, 1612 CAS.
- M. Goto, T. Takahashi, F. Yamashita, A. Koreeda, H. Mori, M. Ohta and Y. Arakawa, Biol. Pharm. Bull., 1997, 20, 1136 Search PubMed.
- N. O. Concha, C. A. Janson, P. Rowling, S. Pearson, C. A. Cheever, B. P. Clarke, C. Lewis, M. Galleni, J.-M. Frère, D. J. Payne, J. H. Bateson and S. S. Abdel-Meguid, Biochemistry, 2000, 39, 4288 CrossRef CAS.
- K. Senda, Y. Arakawa, S. Ichiyama, K. Nakashima, H. Ito, S. Ohsuka, K. Shimokata, N. Kato and M. Ohta, J. Clin. Microbiol., 1996, 34, 2909 CAS.
- Y. Arakawa, N. Shibata, K. Shibayama, H. Kurokawa, T. Yagi, H. Fujiwara and M. Goto, J. Clin. Microbiol., 2000, 38, 40 CAS.
- KaleidaGraph (1997) Synergy Software Inc., 2457 Perkiomen Ave., Reading, PA 19606.
- K. Håkansson, M. Carlsson, L. A. Svensson and A. Liljas, J. Mol. Biol., 1992, 227, 1192 CAS.
- R. F. Chen and J. C. Kernohan, J. Biol. Chem., 1967, 242, 5813 CAS.
- S. K. Nair, J. F. Krebs, D. W. Christianson and C. A. Fierke, Biochemistry, 1995, 34, 3981 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 2nd edn., Kluwer Academic/Plenum, New York, 1999 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and analytical and spectral characterization data. See http://www.rsc.org/suppdata/ob/b2/b209086d/ |
| This journal is © The Royal Society of Chemistry 2003 |
