Preservation of the cluster core upon formation of Ti3O(OPri)8(benzoate)2 from Ti3O(OR)10
Ivan
Mijatovic
,
Guido
Kickelbick
,
Michael
Puchberger
and
Ulrich
Schubert
*
Institute of Materials Chemistry, Vienna University of Technology, Getreidemarkt 9, A-1060, Wien, Austria. E-mail: uschuber@mail.zserv.tuwien.ac.at
First published on 14th November 2002
Abstract
The cluster Ti3O(OPri)8(benzoate)2 is obtained upon reaction of Ti3O(OPri)10 or Ti3O(OPri)9(OMe) with benzoic acid. The remarkable feature of this substitution reaction is that the cluster structure is approximately preserved. Ti3O(OPri)8(benzoate)2 undergoes a dynamic exchange processes of the propoxy groups in solution.
Organically modified transition-metal oxide clusters (OMTOCs) are interesting building blocks for the synthesis of inorganic–organic hybrid materials.1 The most general approach is to use complexing multidentate ligands, such as carboxylates, sulfonates, phosphonates, β-diketonates, etc., to bind the organic groups, which may carry functional groups, directly to the metal atoms at the surface of the cluster.
OMTOCs with multidentate organic ligands can be prepared by two strategies. The multidentate groups can either be grafted to a pre-formed cluster (“surface modification” method) or introduced during the cluster synthesis (“in-situ” method). The latter method has been successfully used for the preparation of a variety of carboxylate- or β-diketonate-substituted metal oxide clusters by reaction of metal alkoxides with carboxylic acids or β-diketones,1 where concomitant ester formation results in the formation of oxo and hydroxo ligands.
The surface modification method is less general. The reason is that the post-synthesis modification of the cluster surface by organic groups requires (i) reactive surface groups, such as OH, Cl or OR, and (ii) the simultaneous balancing of charges and co-ordination numbers upon substitution of these groups. Substitution can therefore only be expected to proceed without major difficulties, if both the number of the occupied co-ordination sites and the charges of the entering ligands are the same as those of the leaving groups. For example, when Ti16O16(OEt)32 was reacted with small proportions of carboxylic acids, a fraction of the bridging OEt groups was replaced by bridging carboxylate groups; the resulting cluster has not been structurally characterized. For higher carboxylate∶Ti proportions, the cluster was degraded.2 However, the presence of bridging alkoxo ligands does not guarantee that substitution by bidentate ligands will take place. Reaction of Ti7O4(OEt)20 with benzoic acid resulted in the formation of the new cluster Ti6O4(OEt)14(OOCPh)2 with concomitant major rearrangement of the cluster core, despite the presence of bridging OR groups in the starting cluster.3 Even complete degradation may occur, as observed for the reaction of Zr4O2(OMc)12 with acetylacetone.4
We now report a rare example where the general structure of a titanium oxo alkoxo cluster was preserved upon substitution of alkoxo ligands by carboxylates. When a toluene solution of the cluster Ti3O(OPri)10 (1a) or Ti3O(OPri)9(OMe) (1b)5 was reacted with 1.5 equivalents of benzoic acid, the new cluster Ti3O(OPri)8(OOCPh)2 (2) was obtained in high yield (eqn. 1). A higher portion of benzoic acid (such as 2 equivalents as required by eqn. 1) leads to a degradation of 2 and to lower yields.
 | (1) |
The molecular structure of 2 was determined by an X-ray structure analysis. Fig. 1 shows the comparison with the structure of the starting cluster 1b (1a is isostructural5). The three titanium atoms in 1 are capped by one oxygen atom and one alkoxy group. Three propoxy groups bridge the edges of the Ti3 triangle, and the remaining propoxy groups are terminally bonded to the titanium atoms. In 2, one of the benzoate ligands (O9/O11) replaces a bridging alkoxy group. According to the general considerations made above, this is an expected reaction because both the co-ordination numbers and charges are preserved. However, the group substituted by the second benzoate ligand (O30/O32) is the μ3 alkoxy group capping the Ti3 triangle. Because a tridentate ligand is replaced by a bidentate ligand with the same charge, one of the co-ordination sites at one of the titanium atoms must be left empty. One titanium atom (Ti2) does indeed change its co-ordination number from 6 to 5.
 | ||
| Fig. 1 A comparison of the structures of Ti3O(OPri)9(OMe) (1b) (left) and Ti3O(OPri)8(OOCPh)2 (2) (right). | ||
Clusters of this structural type were already obtained, and structurally characterized, upon reaction of Ti(ONp)4
(Np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2CMe3) with acetic acid or tert-butylacetic acid;6 the acetate derivative was postulated as an intermediate in the formation of Ti6O4(OPri)12(acetate)4 from Ti(OPri)4 and acetic acid.7
CH2CMe3) with acetic acid or tert-butylacetic acid;6 the acetate derivative was postulated as an intermediate in the formation of Ti6O4(OPri)12(acetate)4 from Ti(OPri)4 and acetic acid.7
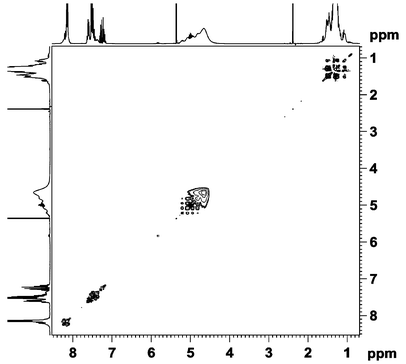
The dynamic behavior of compound 2 in CD2Cl2 solution was studied at different temperatures by 1D and 2D NMR spectroscopy. The proton spectrum at 298 K showed resonances in three distinct regions, i.e. 8.3–7.1 ppm (C6H5), 5.3–4.3 ppm (very broad and unstructured, CH) and 1.6–0.9 ppm (CH3). In the HSQC and HMBC spectra six resolved OiPr signals were observed, five assigned to OiPr groups from the cluster and one from free isopropanol.8 In the EXSY spectrum at room temperature (Fig. 2), exchange signals were observed between all the different OiPr groups.
By cooling the solution to 233 K, a sharpening of the broad proton signals of the CH groups of the OiPr groups was observed. When the solution was further cooled to 193 K, the CH signals of the OiPr groups showed again a broadening and a further splitting. In contrast, the aromatic region remained nearly unchanged. The HSQC (Fig. 3) and HMBC spectra at this temperature showed now signals of eight distinct isopropanolate groups, seven from the cluster and one of the free isopropanol. At this temperature, exchange of the OiPr groups was no longer observed.
 | ||
| Fig. 3 HSQC spectrum of 2 (CD2Cl2) at 193 K (CH region). | ||
Titanium oxo alkoxo clusters (with only oxo and alkoxo substituents) range in size from Ti3O(OR)10 to Ti17O24(OiPr)20.9 A general reactivity pattern emerges. While some of the alkoxo ligands in the bigger clusters (starting with Ti11O13(OiPr)1810) can be replaced by other alkoxo groups, the opposite is true for the exchange of alkoxo groups against carboxylate ligands. We found that only Ti3O(OR)10 undergoes substitution with concomitant preservation of the general cluster structure (this work). Ti7O4(OEt)20 also reacts with benzoic acid, but the cluster structure is completely re-organized.3 When Ti11O13(OiPr)18 is reacted with benzoic acid, complete degradation is observed. The same was observed for Ti12O16(OiPr)16.2
Experimental
All reactions were carried out using standard Schlenk techniques in an argon atmosphere. Argon was purified using Cu and molecular sieve (4 Å). The glass equipment was flame-dried. The solvents were made oxygen-free and water-free by standard methods. The starting clusters 1a and 1b were prepared according to the literature.5 The NMR spectra were recorded on a Bruker Avance 300, 300.13 MHz {1H} and 75.47 MHz {13C}, equipped with a 5 mm broadband head and a z-gradient unit. The 2D spectra were measured according to Bruker standard pulse sequences: HSQC (Heteronuclear Single Quantum Correlation), HMBC (Heteronuclear Multiple Bond Correlation), EXSY (Exchange Spectroscopy); a mixing time of 1 s was used.Synthesis of 2 from 1
The solution of 0.41 g (3.37 mmol) of benzoic acid in 5 ml toluene was added dropwise to a stirred solution of 1.73 g (2.25 mmol) of 1a in 15 ml of toluene. Stirring was continued at room temperature for 14 h, then all volatile compounds were removed in vacuo. The solid residue was dissolved in 4 ml of toluene, and 20 ml of acetonitrile were added. Storage of the solution at −30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C resulted in the crystallization of 2 within two weeks (1.20 g, 61% yield relative to the employed amount of 1a).
°C resulted in the crystallization of 2 within two weeks (1.20 g, 61% yield relative to the employed amount of 1a).
The same cluster was obtained from 0.73 g (1.00 mmol) of 1b and 0.18 g (1.51 mmol) of benzoic acid by the same procedure (0.60 g, 69% yield relative to the employed amount of 1b).
X-Ray crystallography
Single crystal data were collected on a Siemens SMART diffractometer with a CCD area detector (Mo-Kα radiation, λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71.073 pm). The data were corrected for polarization and Lorentz effects, and an empirical absorption correction (SADABS) was applied. The cell dimensions were refined with all unique reflections. The structure was solved by direct methods (SHELXS86). Refinement was carried out with the full-matrix least-squares method based on F2
(SHELXL93) with anisotropic thermal parameters for all non-hydrogen atoms. Hydrogen atoms were inserted in calculated positions and refined riding with the corresponding atom.
71.073 pm). The data were corrected for polarization and Lorentz effects, and an empirical absorption correction (SADABS) was applied. The cell dimensions were refined with all unique reflections. The structure was solved by direct methods (SHELXS86). Refinement was carried out with the full-matrix least-squares method based on F2
(SHELXL93) with anisotropic thermal parameters for all non-hydrogen atoms. Hydrogen atoms were inserted in calculated positions and refined riding with the corresponding atom.
CCDC reference number 188068. See http://www.rsc.org/suppdata/nj/b2/b208255a/ for crystallographic data in CIF or other electronic format.
Crystallographic data for 2: C38H66O13Ti3: M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 874.6, monoclinic, space group P21/c, a
874.6, monoclinic, space group P21/c, a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1191.42(7), b
1191.42(7), b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3793.0(2), c
3793.0(2), c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1140.83(7) pm, β
1140.83(7) pm, β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115.129(1), V
115.129(1), V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4667.6(6)
4667.6(6)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 pm3, Z
106 pm3, Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, T
4, T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294 K, μ
294 K, μ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.555 mm−1, 11
0.555 mm−1, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 463 independent reflections, Rint
463 independent reflections, Rint![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0253, R1
0.0253, R1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0517, wR2
0.0517, wR2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1178.
0.1178.
Acknowledgements
This work was supported by the the Jubiläumsstiftung der Stadt Wien.References
- F. Ribot and C. Sanchez, Comments Inorg. Chem., 1999, 20, 327 Search PubMed; G. Kickelbick and U. Schubert, Monatsh. Chem., 2001, 132, 13 Search PubMed; U. Schubert, Chem. Mater., 2001, 13, 3487 CrossRef CAS; C. Sanchez, G. J. de A. A. Soler-Illia, F. Ribot, T. Lalot, C. R. Mayer and V. Cabuil, Chem. Mater., 2001, 13, 3061 CrossRef CAS; G. Kickelbick, Progr. Polymer Sci. Search PubMed , in press.
- G. J. de A. A. Soler-Illia, L. Rozes, M. K. Boggiano, C. Sanchez, C.-O. Turrin, A.-M. Caminade and J.-P. Majoral, Angew. Chem., Int. Ed., 2000, 39, 4248 CrossRef; G. J. de A. A. Soler-Illia, E. Scolan, A. Loius, P.-A. Albouy and C. Sanchez, New J. Chem., 2001, 25, 156 RSC.
- I. Mijatovic, G. Kickelbick and U. Schubert, Eur. J. Chem., 2001, 1933 Search PubMed.
- B. Moraru, G. Kickelbick, M. Batistella and U. Schubert, J. Organomet. Chem., 2001, 636, 172 CrossRef CAS.
- V. W. Day, T. A. Eberspacher, Y. Chen, J. Hao and W. G. Klemperer, Inorg. Chim. Acta, 1995, 229, 391 CrossRef CAS.
- T. J. Boyle, R. P. Tyner, T. M. Alam, B. L. Scott, J. W. Ziller and B. G. Potter Jr., J. Am. Chem. Soc., 1999, 121, 12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 CrossRef CAS.
104 CrossRef CAS. - N. Steunou, F. Robert, K. Boubekeur, F. Ribot and C. Sanchez, Inorg. Chim. Acta, 1998, 279, 144 CrossRef.
- The sample used for NMR spectroscopy still contained some isopropanol from the preparation procedure. Residual isopropanol is difficult to remove by drying, even in high vacuum, possibly due to hydrogen bonding. Only crystallization yields isopropanol-free samples of 2.
- N. Steunou, G. Kickelbick, K. Boubekeur and C. J. Sanchez, J. Chem. Soc., Dalton Trans., 1999, 3653 RSC.
- V. W. Day, T. A. Eberspacher, W. G. Klemperer and C. W. Park, J. Am. Chem. Soc., 1993, 115, 8469 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2003 |