Synthesis of new iridium complexes and their electrophosphorescent properties in polymer light-emitting diodes
Weiguo
Zhu
,
Chuanzhen
Liu
,
Lijun
Su
,
Wei
Yang
,
Ming
Yuan
and
Yong
Cao
*
Institute of Polymer Optoelectronics Materials and Devices, South China University of Technology, Guangzhou 510640, P. R. China. E-mail: poycao@scut.edu.cn
First published on 6th November 2002
Abstract
Several new iridium complexes with p-substituted 2-phenylpyridine (R-PPy) ligands have been synthesized and characterized. The complexes were incorporated into phosphorescent polymer light-emitting devices using soluble poly[1,4-bis(6′-cyano-6′-methylheptyloxy)phenylene] (CNPPP) as the host and the resultant materials compared with Ir(PPy)3-doped devices. Green electrophosphorescence was observed, with peak emission at about 495 and 515 nm. Among the devices fabricated, highly efficient polymer light-emitting diodes were obtained with CNPPP doped with fac-tris[2-(4-tert-butylphenyl)pyridinato]iridium. An external quantum efficiency of 4.4% (photoluminescence/electroluminescence) and a luminous efficiency of 10 cd A−1 were obtained at 120 cd m−2. These values remain at 4.2% and 10 cd A−1, respectively, at 2500 cd m−2. The improvement is attributed to improved interaction between the guest and host, and to better and more complete energy transfer from the host singlet to the guest triplet state. These results demonstrate that efficient electrophosphorescence is not limited to small molecule organic light-emitting diodes, it can also be achieved in devices made with polymer hosts.
Introduction
Recently, high efficiency organic light-emitting diodes (OLEDs) have been fabricated using electrophosphorescent molecules such as 2,3,7,8,12,13,17,18-octaethyl-21H,23H-porphyrinatoplatinum (PtOEP),1,2fac-tris(2-phenylpyridinato)iridium [Ir(PPy)3]3–5 and bis{2-[2′-benzo(4,5-a)thienyl]pyridinato-N,C3}iridium acetylacetonate [(Btp)2Ir(acac)].6,7 This has heralded a breakthrough in improving external quantum efficiency and luminous efficiency in OLEDs. Unlike fluorescence-based light-emitting diodes (LEDs), the quantum efficiency of phosphorescence-based LEDs are not limited and can theoretically reach 100% by using both singlet and triplet excitons. As a result, they have attracted attention worldwide, and are considered to be promising candidates for realizing commercial full color LED displays.Much effort has been focused on developing highly efficient phosphorescent guest materials, selecting appropriate host materials and designing efficient device structures. At present, most of the phosphorescent guest materials investigated are complexes of heavy metals, as strong spin–orbit coupling leads to singlet–triplet state mixing, thus resulting in high efficiency electrophosphorescence in LEDs. Ortho-metalated complexes with d6 and d8 metal ions such as Pt(II),2 Os(II),8 and Ir(III)3–7,9 have been extensively investigated for electrophosphorescent device applications. An energy efficiency of 40 lm W−1 and an external quantum efficiency of 15% were reported in OLEDs using an iridium complex as the phosphorescent emitter.3
Research into host materials has mainly focused on small molecules. Typically, small molecule blue emitters such as tris(8-hydroxyquinolinato)aluminium (Alq3),1 4,4′-dicarbazolebiphenyl (CBP),3,4 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP),3,4 1,3-bis(tert-butylphenyl)-1,3,4-oxadiazole (OXD7),7 3-phenyl-4-(1′-naphthyl)-5-phenyl-1,2,4-triazole (TAZ)3,7 have been used in electrophosphorescent devices. In contrast, there are only a few reports on phosphorescence-based LEDs using a polymer as the host material. Peak quantum efficiencies of 1.9 and 3.5% have been reported for a Ir(PPy)3-doped polyvinylcarbazole device10 and a PtOEP-doped polyfluorene device, respectively.11 The quantum efficiencies for phosphorescent dye-doped polymer light-emitting diodes (PLED) prepared so far are much lower than those of dye-doped OLEDs using small molecules as host.
It is of interest to use polymers instead of organic small molecules as host materials, since PLEDs have the potential to be used for large area displays which can be made using simpler processes and at a lower cost. In order to improve performance of phosphorescence-based PLEDs and decrease phase segregation between host and guest, it is very important to design highly efficient phosphorescent guest materials.12 In this paper, we report the synthesis of three new iridium complexes (Scheme 1) which show high phophorescence efficiencies, and report their optical and electroluminescent (EL) properties. We found that altering the length of the of alkyl substitution on the phenylpyridine ligands could lead to significant differences in EL performance.
 | ||
| Scheme 1 Synthetic routes to and chemical structures of the materials used herein. | ||
Experimental details
Reagents
p-tert-Butylbromobenzene, 2-bromopyridine, n-butyllithium, 4-bromophenol, and anhydrous zinc chloride were purchased from Aldrich. Tetrakis(triphenylphosphine)palladium was obtained from TCI Co. Bromobenzene and anhydrous aluminium chloride were used as received. Anhydrous tetrahydrofuran was distilled over sodium–benzophenone under nitrogen prior to use, CS2 was distilled over calcium chloride. 4-(3′,7′-Dimethyloctyloxy)bromobenzene, decanoyl chloride, 1-bromo-4-decylbenzene, and 2,5-dichloro-1,4-bis(6′-cyano-6-′methylheptyloxy)benzene were prepared following published procedures.13–16Syntheses
Instrumentation
All GC-MS data were obtained using a Finnigan Trace GC-MS-2000 Series system. All NMR spectra were acquired with a Bruker Dex-400NMR instrument, using CDCl3 as a solvent. Elemental analysis was performed on a Harrios elemental analyzer. The UV-Visible absorption spectra of films containing iridium complexes were recorded using an HP-8453 UV-Visible system. Photoluminescence (PL) and electroluminescence (EL) spectra were obtained with an Oriel InstaSpec IV CCD system. The PL quantum efficiencies of the blends as solid thin films on quartz substrates were measured with a LabSphere IS80 integrating sphere, together with a UDT S370 digital photometer, according to the method described by Greenham et al.17 Excitation for the measurement of PL spectra and PL efficiency was achieved using the 325 nm line from a He–Cd laser (Melles Griot).Device fabrication and characterization
The polymer light-emitting diodes were fabricated on commercial indium tin oxide (ITO) substrates with a sheet resistance of 15 Ω □−1 (Nanbo, Shengzhen, China). Before spin coating, the ITO substrates were cleaned using acetone, detergent, deionized water, and 2-propanal, then treated with oxygen plasma for 10 min. The LED structure is illustrated in Scheme 2. A 40 nm layer of polyvinylcarbazole (PVK) was spin cast on top of the ITO substrate. The PVK layer functions as a hole-injection layer. A 70 nm phosphorescent dye-doped emitting layer was spin coated on top of the PVK layer. The thickness of the PVK and emitting layers were measured using a Tencor Alpha-step 500 surface profiler. Devices were made with a thin layer of barium as the cathode, deposited using vacuum vapor deposition at a pressure below 3 × 10−4 Pa. Immediately after deposition of Ba, a 200 nm capping layer of aluminium was deposited on top of the Ba metal layer. The cathode area defines the active area of the device, which is typically 0.15 mm2 for the devices in this study. The deposition speed and the thickness of the barium and aluminium layers were monitored with a Sycon Instruments Model STM-100 thickness/rate meter. All steps except the PVK-coating were performed in N2-filled dry boxes with oxygen and water contents of less than 1 ppm. I–V Characteristics were measured with a computerized Keithley 236 source measuring unit. The luminance of the devices was measured with a calibrated photodiode. | ||
| Scheme 2 Schematic representation of the PLED structure. | ||
Results and discussion
Synthesis of iridium complex
The synthesis of the ligands and iridium complexes is depicted in Scheme 1. The 2-arylpyridine ligands were synthesized via Pd-catalyzed cross-coupling using the modified method reported previously.18 The use of zinc (intermediate electronegativity) rather than lithium (low electronegativity) leads to favorable results in Pd-catalyzed coupling between aryl and pyridyl. The reaction was carried out in one step with a yield of over 50%. Iridium complexes were prepared according to the procedure reported previously.19 The reaction between arylpyridines and tris(acetylacetonato)iridium in refluxing glycerol can afford cycloiridium complexes. The yields of about 55% were obtained by varying the ratio of 2-arylpyridine to tris(acetylacetonato)iridium, using a higher ratio than that reported for fac-tris(2-phenylpyridinato)iridium in the literature.19 The elemental analysis results for the complexes are consistent with the structures shown in Scheme 1.Optical and photoluminescent properties
Fig. 1 shows the UV-Visible absorption spectra of three complexes in solid films on quartz substrates. All three iridium complexes have almost identical UV-Visible absorption spectra, similar to that of Ir(PPy)3, at a wavelength from 200 to 800 nm. An intense absorption band is observed in the ultraviolet parts of the spectra from 200 to 400 nm. The intense absorption band around 290 nm can be assigned to a spin-allowed 1π–π* transition on the cyclometalated ligands, and the broad absorption band at lower energy (380 nm) is typical for spin-allowed metal to ligand charge-transfer (1MLCT) transitions, as has been discussed in the literature.19 The 380 nm peak for Ir(DecOPPy)3 is slightly blue-shifted compared with those due to Ir(DecPPy)3 and Ir(BuPPy)3. This indicates that modification of the PPy molecule by adding alkyl or alkoxy groups to the phenyl ring has little influence on the absorption properties of the corresponding iridium complexes. | ||
| Fig. 1 UV-Visible absorption spectra of films of the iridium complexes. | ||
Fig. 2(a) shows the normalized photoluminescence (PL) spectra of Ir(BuPPy)3-doped CNPPP devices with dopant concentrations of 0, 1, 2, 4, and 8 wt%. The photoluminescence profile contains two peaks: one is centered at 430 nm, resulting from the emission of the CNPPP host and the other, observed at 515 nm, is due to Ir(BuPPy)3 triplet emission.20 The emission at 430 nm decreases significantly as the dopant concentration is increased. At dopant concentrations of 4 wt% or more, the PL profiles are dominated by the 515 nm triplet emission. The observed concentration dependence of the PL profile resembles that observed for Ir(PPy)3-doped CNPPP films.12 The results indicate that Forster transfer from CNPPP singlets takes place. In order gain a greater understanding of the energy transfer in such blend films, the absolute PL quantum efficiencies of thin (100 nm) blend films were measured in the integrating sphere, according to the method proposed by Greenham et al.17Table 1 lists the PL efficiencies of blend films containing the four Ir complexes in 4 wt% dopant concentrations (the point at which the emission of the Ir complex becomes dominant over that of CNPPP). For the purposes of comparison, the PL efficiencies of undoped CNPPP film and pure Ir complexes were also measured (Table 1). As expected, the PL efficiencies of the Ir complexes are only ca. 1%, due to concentration quenching. This value is consistent with the results reported for Ir complexes with other ligands.21 As can be seen from Table 1, the PL efficiency varies with alkyl chain length; Ir(BuPPy)3-doped CNPPP shows the highest PL quantum efficiency among the four blends, which is consistent with the electroluminescence (EL) results (see below). There are several possible explanations for the dependence of the PL properties on the alkyl chain length, and further photophysics studies are necessary to obtain a clearer picture.
 | ||
| Fig. 2 (a) PL spectra of Ir(BuPPy)3-doped CNPPP films on quartz substrates. (b) EL spectra of Ir(BuPPy)3-doped CNPPP devices. | ||
| Material | Dopant concentration/wt% | PL efficiency (%) |
|---|---|---|
| Ir(PPy)3–CNPPP | 4 | 32.1 |
| Ir(BuPPy)3–CNPPP | 4 | 41.7 |
| Ir(DecPPy)3–CNPPP | 4 | 28.4 |
| Ir(DecOPPy)3–CNPPP | 4 | 12.3 |
| CNPPP | 0 | 26.3 |
| Ir(PPy)3 | — | 1.1 |
| Ir(BuPPy)3 | — | 1.0 |
Device performance
Polymer LEDs were fabricated from the Ir complex-doped CNPPP films. A schematic representation of the device structure is shown in Scheme 2. Since the highest occupied molecular orbital (HOMO) level of alkoxy-substituted PPPs is 5.7 eV,15 a 40 nm polyvinylcarbazole film was placed between the indium tin oxide and the emitting layer to facilitate hole injection. A thin layer of Ba with a 200 nm Al capping layer was used as the cathode. The thickness of the Ir complex–polymer blend was 70–80 nm in all cases. Fig. 2(b) shows the corresponding EL spectra obtained from the devices with different Ir(BuPPy)3 dopant concentrations. In contrast to the PL spectra [Fig. 2(a)], which show a significant contribution from the CNPPP host at low dopant concentrations, no difference can be seen between the EL emission profiles for devices with dopant concentrations between 1 and 8 wt%. The spectra of the devices contain only emissions from the Ir complexes, even at dopant concentrations as low as 1 wt%. This result clearly indicates that Forster transfer is not the only mechanism responsible for EL performance. O'Brien et al.11 suggested that there is a significant contribution from carrier trapping. Recently, Lane et al. investigated Pt complex-doped polyfluorene and concluded that Forster transfer of singlet excitons was weak and the dominant emission mechanism in their doped polymer LEDs was charge trapping followed by recombination on the Pt complex.22 McGhee et al. reported a large difference between the PL and EL spectra of Eu complex–CNPPP devices at low dopant concentrations.23 They attributed this behavior to the difference in recombination zone for photo- and electrical excitation.Fig. 3. compares the EL spectra of devices prepared with CNPPP doped with different Ir complexes at 4 wt% dopant concentration. No emission peak at 420 nm corresponding to the host is observed, indicating that efficient energy transfer from CNPPP to the iridium complexes occurs at a dopant concentration of 4 wt% for all these devices. The Ir(BuPPy)3 device emits green light centered at 515 nm, with exactly the same peak position and emission spectrum as for the Ir(PPy)3 device. It is interesting to note that the attaching decyl and decyloxy groups at the p-position of the phenyl ring of 2-phenylpyridine shifts the emission peak significantly into the blue region. Both the Ir(DecOPPy)3 and Ir(DecPPy)3 devices emit blue–green light centered at 495 nm.
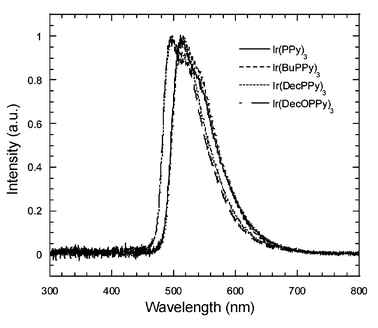 | ||
| Fig. 3 EL spectra of devices containing CNPPP doped with various iridium complexes. | ||
Table 2 shows the device performance of the three new complexes along with Ir(PPy)3 in 4 wt% dopant concentration. Attaching a butyl group at the p-position of the phenyl ring of 2-phenylpyridine improves the device performance. The Ir(BuPPy)3-doped CNPPP device shows a generally higher quantum efficiency (QE) than the Ir(PPy)3-doped device at each dopant concentration. For the 4 wt% Ir(BuPPy)3 device, an external quantum efficiency of 4.2% (PL/EL) and a luminous efficiency of 10 cd A−1 were obtained at 2500 cd m−2, while for the Ir(PPy)3-doped device fabricated under the same experimental conditions, the values are only 2.3% and 5.4 cd A−1, respectively, at 1770 cd m−2 (Table 2). Devices prepared using Ir(DecOPPy)3 and Ir(DecPPy)3 show much lower efficiencies than the Ir(PPy)3 device, as can be seen from Table 2. It is important to note that the reduction rate of the QE under higher bias voltages (and currents) for the devices with different substitutions of the phenyl ring in 2-phenylpyridine differ remarkably. It is well known that the external QE in Ir(PPy)3–TAZ devices3 reaches its maximum (15%) at very low current densities (<10−2 mA cm−2) and decays very quickly with increasing current density due to triplet–triplet annihilation.3Fig. 4 compares the luminous efficiencies of devices prepared from Ir(PPy)3-, Ir(BuPPy)3-, and Ir(DecOPPy)3-doped CNPPP at a dopant concentration of 2 wt%. The Ir(BuPPy)3 device again shows the best performance at high current densities. For one of the best Ir(BuPPy)3-doped CNPPP devices with a dopant concentration of 4 wt%, the external QE and EL efficiency were 4.4% and 10 cd A−1, respectively, at 120 cd m−2. The efficiencies remain at 4.2% and 10 cd A−1, respectively, at 2500 cd m−2. A maximum external QE of 5.1% and an EL efficiency of 12 cd A−1 were observed at 800 cd cm−2 and at a current density of 6.8 mA cm−2. According to Greenham et al.,24 the internal quantum efficiency can be estimated from the optical power emitted in the forward direction. Assuming the refractive index of the substituted polyphenylene as 1.6, as observed for most conjugated polymers,25 the maximal internal quantum efficiency is 22% for the Ir(BuPPy)3-doped CNPPP devices. Better blending and more homogeneous distribution of the guest molecules in the host polymer matrix may account for the improved energy transfer and the suppression of triplet–triplet annihilation.
 | ||
| Fig. 4 Luminous efficiency vs. bias for devices prepared with blend films with 2 wt% dopant concentration. | ||
| Guest | V/V | Device performance | |||
|---|---|---|---|---|---|
| I/mA | Luminance/cd m−2 | QE (%) | EL efficiency/cd A−1 | ||
| Ir(PPy)3 | 30 | 4.7 | 1668 | 2.27 | 5.4 |
| Ir(BuPPy)3 | 30 | 3.8 | 2501 | 4.22 | 10.0 |
| Ir(DecOPPy)3 | 28 | 7.5 | 1717 | 1.46 | 3.5 |
| Ir(DecPPy)3 | 26 | 2.7 | 1022 | 1.39 | 3.3 |
Overall, these are very encouraging results. Although the external quantum efficiency for phosphorescent dye-doped PLEDs is still much lower than the corresponding OLEDs,3,26 significant improvement in QE reduction rate with increasing current density breaks the barrier to phosphorescent PLEDs being used for passive displays operated at low duty cycles. These results also demonstrate that efficient electrophosphorescence is not specific to small molecule OLEDs, it can also be achieved in devices made with a polymer host. At this stage of our experiments we don't know why the longer 2-phenylpyridine chain substitution in Ir(DecOPPy)3 and Ir(DecPPy)3) leads to lower QEs than both Ir(PPy)3 and Ir(BuPPy)3. However, this fact clearly indicates that there are many factors which affect the energy transfer process in such systems. An overlap of the emission spectrum of the host and the absorption spectrum of the guest is considered to be an important condition to obtain high efficiency in phosphorescent dye-doped LEDs. In this study, all of these iridium complexes have the same overlap between the emission spectrum of the CNPPP host and their absorption spectra, similar to that for Ir(PPy)3. Nevertheless, the device performance is very different in each case. These results suggest that the energy transfer from the blue light-emitting host to the phosphorescent dye is a complicated process. Overlap of the emission of the host and the absorption of the guest (Fig. 1) is a necessary condition, but not a sufficient one. Other processes need to be considered. One such is the matching of the LUMO and HOMO levels between the host and guest in the singlet and triplet states. Another is phase segregation between the crystalline guest molecules and the amorphous host polymer. We have designed a series of experiments to identify the underlying mechanism and these are currently in progress.
Conclusions
We have synthesized and characterized three new iridium complexes with different substituted PPy ligands. The EL performance of these three complexes was compared with that of Ir(PPy)3 in the same device configuration and using the same fabrication conditions. We found that device performance is quite sensitive to the substitution on the 2-phenylpyridine ligand. Highly efficient PLEDs were obtained with Ir(BuPPy)3-doped CNPPP. An external QE of 4.4% PL/EL and a luminous efficiency of 10 cd A−1 were obtained at 120 cd m−2. These values remain at 4.2% and 10 cd A−1, respectively, at 2500 cd m−2. We attribute these improvements over other similar devices to improved interaction between the guest and host, and to better and more complete energy transfer from the host singlet to the guest triplet state. These results demonstrate that efficient electrophosphorescence is not only a feature of small molecule OLEDs, it can also be achieved in devices made with a polymer host. This approach allows high efficiency electrophosphorescent devices fabricated via simpler and more cost-efficient processes, such as ink-jet printing and screen printing.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Project No. 29992530-6) and the Postdoctoral Science Foundation of China (Project No. 2001-5).References
- D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature., 1998, 395, 151 CrossRef CAS.
- D. E. O'Brien, M. A. Baldo, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 74, 442 CrossRef.
- C. Adachi, M. A. Baldo, S. R. Forrest and M. E. Thompson, Appl. Phys. Lett., 2000, 77(6), 904 CrossRef CAS.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75(1), 4 CrossRef CAS.
- Ot. Watarabe, K. Nakamura, S. Kawami and Y. Fukuda, Synth. Met., 2001, 122, 203 CrossRef.
- C. Adachi, M. A. Baldo, S. R. Forrest, S. Lamansky, M. E. Thompson and R. C. Kwong, Appl. Phys. Lett., 2001, 78(11), 1622 CrossRef CAS.
- M. E. Thompson, S. Lamansky, P. Djurovich, D. Murphy and F. A. Razzaq, SID Proc., 2000, 337 Search PubMed.
- S. Lamansky, P. Djurovich, D. Murphy, F. A. Razzaq, H. E. Lee, C. Adachi, P. Burrough, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2001, 123, 4304 CrossRef CAS.
- Y. Ma, H. Y. Zhang, J. C. Shen and C. Che, Synth. Met., 1998, 94, 245 CrossRef CAS.
- C. L. Lee, K. B. Lee and J. J. Kim, Appl. Phys. Lett., 2000, 77(15), 2280 CrossRef CAS.
- D. E. O'Brien, C. Giebler, R. B. Flecher, J. Cadlby, L. C. Palilis, D. G. Lidzey, P. A. Lane, D. D. C. Bradley and N. Blan, Synth. Met., 2001, 116, 379 CrossRef CAS.
- W. Zhu, Y. Mo, M. Yuan, W. Yang and Y. Cao, Appl. Phys. Lett., 2002, 80, 2045 CrossRef CAS.
- H. Spreitzer, W. Krreuder, H. Becker, E. Kluge, H. Schoo and R. Demandt (PCT), WO Pat. Appl., 98/27136, 1996 Search PubMed.
- G. J. Solan and W. R. Vaughan, J. Org. Chem., 1957, 22, 750 CrossRef.
- G. W. Gray, M. Hird, D. Lacey and K. J. Toyne, J. Chem. Soc., Perkin Trans. 2, 1989, 2041 RSC.
- Y. Yang, Q. Pei and A. J. Heeger, J. Appl. Phys., 1996, 79, 934 CrossRef CAS.
- N. C. Greenham, I. D. W. Samuel, G. R. Hayes, R. T. Phillips, Y. A. R. R. Kessener, S. C. Moratti, A. B. Holmes and R. H. Friend, Chem. Phys. Lett., 1995, 241, 89 CrossRef CAS.
- E. Negishi, F. T. Luo, R. Frisbee and H. Matsushita, Heterocycles, 1982, 18, 117 Search PubMed.
- Y. Zhang, C. D. Baer, C. C. Neto, P. O'Brien and D. A. Sweigart, Inorg. Chem., 1991, 30, 1685 CrossRef CAS.
- M. G. Colombo, T. C. Brunold, T. Riedener and H. U. Gudel, Inorg. Chem., 1994, 33, 545 CrossRef CAS.
- X. Gong, M. R. Robinson, J. C. Ostrowski, D. Moses, G. C. Bazan and A. J. Heeger, Adv. Mater., 2002, 14, 581 CrossRef CAS.
- P. A. Lane, L. C. Palilis, D. F. O'Brien, C. Giebler, A. J. Cadby, D. G. Lidzey, A. J. Cambell, W. Blau and D. D. C. Bradley, Phys. Rev. B, 2002, 63, 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235
235![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 206.
206. - M. D. McGhee, T. T. Bergstedt, C. Zhang, A. P. Saab, M. B. O'Regan, G. C. Bazan, V. I. Srdanov and A. J. Heeger, Adv. Mater., 1999, 11, 1349 CrossRef CAS.
- N. C. Greenham, R. H. Friend and D. D. C. Bradley, Adv. Mater., 1994, 6, 491 CrossRef CAS.
- I. D. Parker and Q. Pei, Appl. Phys. Lett., 1994, 65, 1272 CrossRef CAS.
- M. A. Baldo, M. E. Thompson and S. R. Forrest, Nature, 2000, 403, 750 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
