Direct formation of 2,4-disubstituted tetrahydropyranols in water mediated by an acidic solid resin
Charlene C. K. Keh and Chao-Jun Li*
Tulane University, Department of Chemistry, New Orleans, Louisiana, USA. E-mail: cjli@tulane.edu; Fax: 504-865-5596; Tel: 504-865-5573
First published on 20th December 2002
Abstract
Amberlite® IR-120 Plus resin, a readily regenerated acidic solid resin, mediates the formation of tetrahydropyranol derivatives in water. Various aldehydes were reacted with homoallyl alcohol under the reaction conditions to yield the desired tetrahydropyranol derivatives in moderate to good yields.
Green ContextAchieving the cleaner synthesis of organic compounds can be done in several ways including VOC solvent avoidance, the use of heterogeneous catalysts and atom economical reactions. By combining these greener methods we can move towards the ‘ideal synthesis’ whereby auxiliaries, energy, resources and waste are minimised. Here we see a nice example of a cleaner synthesis involving multiple improvements. Thus an atom economical synthesis of tetrahydropyranols is carried out in water, using a recoverable heterogeneous catalyst.JHC |
Tetrahydropyrans are a prevalent subunit encountered in many natural products such as carbohydrates, polyether antibiotics and marine toxins.1 The Prins cyclization,2 which entails the formation of a C–C bond, is an important method in the formation of tetrahydropyran derivatives. Initially discovered in 1899, it consisted of the condensation of olefins with aldehydes under strongly acidic conditions and high reaction temperatures which limited its potential as an effectual synthetic methodology. Currently there has been an actualization of the potentiality in the utilization of the Prins cyclization for the formation of tetrahydropyran derivatives in a stereocontrolled manner.3 However, in keeping with our interest in green chemistry4 methodology, we were interested in exploring the possibility of running the reaction in water at a lower temperature while utilizing a commercially available solid acid5 source which could be easily filtered from the reaction media.
Previously, we reported our investigation6 into the efficacy of the Prins cyclization utilizing InCl3, which is a mild Lewis acid, relative to the stronger Lewis acid catalysts (e.g. TiCl4, SnCl4) previously developed. However, the usage of a Lewis acid with a nucleophilic anion resulted in the formation of 4-halotetrahydropyran derivatives (Taddei–Chan method),7 which is not as synthetically useful as 4-tetrahydropyranol derivatives. Recently, we have found that the usage of a catalytic amount of scandium triflate,8 a Lewis acid with a non-nucleophilic anion, allowed for the formation of oxygenated tetrahydropyran derivatives in refluxing chloroform.9
However, one of the primary and fundamental objectives in our methodology development, has always been to incorporate effective methodology with the principles of green chemistry. The direct formation of the desired and synthetically valuable derivative is in accordance with our green chemistry objective in two ways: (1) usage of an environmentally benign solvent and (2) elimination of an additional step required to convert from the halogenated to the oxygenated derivative. To this end, we recently reported the direct formation of tetrahydropyranol derivatives using ionic liquid as the reaction media in the presence of a catalytic amount of Lewis acid.10
Herein, we wish to report the direct formation of tetrahydropyranol derivatives in water using the Amberlite® IR-120 Plus resin—an acidic resin with a sulfonic acid moiety. A mixture of an aldehyde and homoallyl alcohol in water, in the presence of the resin and under sonication, yielded the desired tetrahydropyranol derivatives (Fig. 1).† However, without sonication, the reaction does not proceed.11 This is the first example we are aware of that allows for the direct selective formation of tetrahydropyranol derivatives in water. The merits of this methodology are threefold: (1) the resin can be easily removed from the reaction mixture by filtration and reused;12 (2) water, the solvent of choice, is relatively economical and most environmentally friendly; and (3) the overall reaction is a cross-molecular isomerization that is atom-economical.13
 | ||
| Fig. 1 Formation of tetrahydropyranol derivatives. | ||
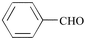
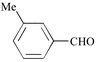
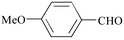
As shown in Table 1, the overall yields of the various aldehydes are good. Due to a solubility factor, when the aldehyde used is a solid (i.e., entries 3, 6, 7, 8, 9), the conversion rates14 are lower relative to the liquid aldehydes (i.e., entries 1, 2, 4, 5, 10, 11).
A tentative mechanism for the direct tetrahydropyranol formation is shown in Scheme 1. Initially, the acidic resin activates the aldehyde via protonation, followed by a nucleophilic attack of the activated carbonyl by the homoallyl alcohol to generate the hemiacetal. A Prins-type cyclization accompanied by the quenching of the resultant carbocation by water furnishes the desired tetrahydropyranol. The surface of the resin may provide an environment that prevents competing by-product formation. Selective formation of the cis isomer is mostly due to thermodynamic control.
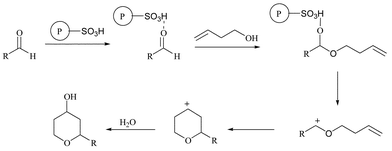 | ||
| Scheme 1 | ||
In summary, we have developed a method for the direct formation of synthetically useful tetrahydropyranol derivatives in water. We believe that there are many additional applications of this resin and further investigation into its utilization will result in its expanded and increased usage as an alternative acid source in aqueous media.
This work was supported by the NSF CAREER Award, NSF-EPA Joint Program for a Sustainable Enviroment, and a Louisiana Board of Regents Graduate Fellowship (C. C. K. K.).
Notes and references
- (a) Polyether Antibiotics, ed. J. W. Westly, Marcel Dekker, New York, 1983, vol. I and II Search PubMed; (b) K. C. Nicolaou and E. J. Sorensen, Classics in Total Synthesis, VCH, Weinheim, 1996 Search PubMed.
- For reviews of the Prins reaction, see: (a) E. Arundale and L. A. Mikeska, Chem. Rev., 1952, 51, 505 CrossRef CAS; (b) D. R. Adams and S. R. Bhatnagar, Synthesis, 1977, 661 CrossRef CAS; (c) B. B. Snider, in Comprehensive Organic Synthesis, ed. B. M. Trost, Pergamon, Oxford, 1991, vol. 2. pp 527–561 Search PubMed.
- For representative recent examples, see: (a) I. E. Markó, A. P. Dobbs, V. Scheirmann, F. Chellé and D. J. Bayston, Tetrahedron Lett., 1997, 38, 2899 CrossRef CAS; (b) S. D. Rychnovsky, G. Yang, Y. Hu and U. R. Khire, J. Org. Chem., 1997, 62, 3022 CrossRef CAS; (c) S. D. Rychnovsky, Y. Hu and B. Ellsworth, Tetrahedron Lett., 1998, 39, 7271 CrossRef CAS; (d) V. V. Samoshin, D. E. Gremyachinskiy and P. H. Gross, Mendeleev Commun., 1999, 53 CrossRef; (e) M. J. Cloninger and L. E. Overman, J. Am. Chem. Soc., 1999, 121, 1092 CrossRef CAS; (f) C. Chen and P. S. Marino, J. Org. Chem., 2000, 65, 3252 CrossRef CAS; (g) D. J. Kopecky and S. D. Rychnovsky, J. Am. Chem. Soc., 2001, 123, 8420 CrossRef CAS; (h) J. J. Jaber, K. Mitsui and S. D. Rychnovsky, J. Org. Chem., 2001, 66, 4679 CrossRef CAS; (i) S. R. Crosby, J. R. Harding, C. D. King, G. D. Parker and C. L. Willis, Org. Lett., 2002, 4, 577 CrossRef CAS.
- P. T. Anastas and J. C. Warner, Green Chemistry, Oxford, New York, 1998 Search PubMed.
- For an excellent review on the usage of solid acids in green chemistry, see: J. H. Clark, Acc. Chem. Res., 2002, 35, 791 Search PubMed.
- (a) X. F. Yang, J. T. Mague and C. J. Li, J. Org. Chem., 2001, 66, 739 CrossRef CAS; (b) J. Yang, G. S. Viswanathan and C. J. Li, Tetrahedron Lett., 1999, 40, 1627 CrossRef CAS; (c) G. S. Viswanathan, J. Yang and C. J. Li, Org. Lett., 1999, 1, 993 CrossRef.
- For other examples for the formation of 4-halo derivatives, see: (a) Z. Y. Wei, J. S. Li, D. Wang and T. H. Chan, Tetrahedron Lett., 1987, 38, 3441 CrossRef CAS; (b) L. Coppi, A. Ricci and M. Taddei, Tetrahedron Lett., 1987, 28, 973 CrossRef CAS; (c) L. Coppi, A. Ricci and M. Taddei, J. Org. Chem., 1988, 53, 913 CrossRef; (d) Z. Y. Wei, D. Wang, J. S. Li and T. H. Chan, J. Org. Chem., 1989, 54, 5768 CrossRef CAS.
- (a) S. Kobayashi and I. Hachiya, J. Org. Chem., 1994, 59, 3590 CrossRef CAS; (b) S. Kobayashi, in Organic Synthesis in Water, ed. P. A. Greico, Thomson Science, Glasgow, 1998 Search PubMed.
- (a) W. C. Zhang, G. S. Viswanathan and C. J. Li, Chem. Commun., 1999, 291 RSC; (b) W. C. Zhang and C. J. Li, Tetrahedron, 2000, 56, 2403 CrossRef CAS.
- C. C. K. Keh, V. V. Namboodiri, R. S. Varma and C. J. Li, Tetrahedron Lett., 2002, 43, 4993 CrossRef CAS.
- The energy usage of sonication was not calculated.
- From the technical report regarding this resin supplied from Aldrich, the resin can be regenerated using 1–10% sulfuric acid.
- (a) B. M. Trost, Science, 1991, 254, 1471 CAS; (b) B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259 CrossRef CAS.
- During our optimization of the reaction, we found that longer reaction times did not result in a higher conversion.
Footnote |
| † General procedure for the reaction: to a solution of resin (3 g) in 2.5 mL H2O, was added benzaldehyde (147 mg, 1.39 mmol) and the reaction mixture allowed to sonicate (commercial Branson sonicator, 100 W) at room temperature for 20 min. The homoallyl alcohol (50 mg, 0.693 mmol) was then added and the reaction was monitored by TLC. Upon completion (2 days), the resin was filtered off, washed with water (5 mL) and the aqueous layer was extracted with diethyl ether (5 mL × 5). The crude mixture was concentrated in vacuo and purified using column chromatography on silica gel with eluent (2∶1 hexanes–ethyl acetate) to yield the major product (97 mg, 87%). |
| This journal is © The Royal Society of Chemistry 2003 |