Brønsted acidic ionic liquid 1-methylimidazolium tetrafluoroborate: a green catalyst and recyclable medium for esterification
Hua-Ping Zhu, Fan Yang, Jie Tang* and Ming-Yuan He*
Center for the Chemistry of Ionic Liquids, Department of Chemistry, East China Normal University, Shanghai, 200062, China. E-mail: jietang@mail.ecnu.edu.cn
First published on 11th December 2002
Abstract
Esterification of carboxylic acids with alcohols could be carried out in a Brønsted acidic ionic liquid 1-methylimidazolium tetrafluoroborate ([Hmim]+BF4−). Good yields were obtained, and the esters produced could be easily separated from the reaction mixture without any volatile organic solvents. The ionic liquid [Hmim]+BF4− could be reused after removal of water.
Green ContextEsterification is a simple process, yet there are still many challenges to produce a simple and clean route to esters from acids and alcohols. One particularly attractive method uses a Brønsted acidic ionic liquid to carry out the reaction. The product ester is insoluble and separates from the ionic liquid as the reaction proceeds, presumably helping to drive the equilibrium towards products. Very high yields can be achieved with a simple separation, and the ionic liquid can be reused several times.DJM |
Introduction
Organic esters are important products or intermediates in the chemical and pharmaceutical industries.1 Esterification of carboxylic acids using liquid inorganic acid catalysts is well known.2–4 However, removal of water or/and use of excess amount of the reactants is generally needed for satisfactory conversion, and a large volume of volatile organic solvents is often required. Meanwhile, it is very difficult to recycle the liquid inorganic acid catalysts, which need to be neutralized after the reaction. Large amounts of volatile organic solvents and liquid inorganic acids may result in pollution to the environment.Ionic liquids are emerging as green reaction media for a variety of organic transformations.5 Recently, it has been reported that esterification could be carried out in ionic liquids, such as 1-hexyl-3-methylimidazolium hydrogen sulfate and 1-[2-(2-hydroxyethoxy)ethyl]-3-methylimidazolium hydrogen sulfate.6 However, the preparation of these ionic liquids was not convenient, and volatile organic solvents were also used for the preparation and reuse of the ionic liquids. Furthermore, the conversions were lower than 60% for most of the substrates.
In this paper a practical and efficient procedure for esterification of carboxylic acids with alcohols is reported, using a Brønsted acidic ionic liquid, 1-methylimidazolium tetrafluoroborate ([Hmim]+BF4−), as recyclable catalyst and solvent (Scheme 1).
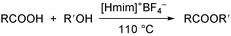 | ||
| Scheme 1 | ||
Experimental
General remarks
All commercial chemicals were used as received. The reactions were carried out in a glass reactor fitted with a reflux condenser and oil-bath. The reacting acids and alcohols (indicated in Table 1) were added to the ionic liquid [Hmim]+BF4−. The esterification reactions proceed for a period of time ranging from 2 to 10 h with vigorous stirring and using an oil-bath at 110 °C. Reaction progress was monitored by GC-MS (Agilent GC: 6890 MS: 5973N). After the reaction, the ester and ionic liquid [Hmim]+BF4− were separated conveniently by decanting. The ionic liquid [Hmim]+BF4− was reused after removal of water under vacuum (0.01 Torr) at 70 °C for 1 h.Preparation of ionic liquid 1-methylimidazolium tetrafluoroborate ([Hmim]+BF4−)7
1-Methylimidazole (61.5 g, 0.75 mol) was placed in a three-necked flask, which was provided with a stirrer and cooled to 0 °C. Then tetrafluoroboric acid (0.75 mol, 40% solution in water) was added slowly over a period of 30 min while stirring and cooling to maintain the temperature at 0–5 °C. The reaction mixture was stirred for an additional period of 2 h. Water was removed in vacuum to give the product as a colorless liquid, which solidified on cooling.Typical esterification procedure
1-Butanol (2 ml, 0.02 mol), equivalent acetic acid (1.6 ml, 0.02 mol) and ionic liquid [Hmim]+BF4− (2 ml) were added in a flask with a reflux condenser and oil-bath. The reaction mixture was stirred for 2 h with the oil-bath at 110 °C. Reaction progress was monitored by GC-MS. After reaction, the mixture was decanted, the ester was isolated, and the ionic liquid [Hmim]+BF4− was reused after removal of water under vacuum (0.01 Torr) at 70 °C for 1 h.Results and discussion
The preparation of the ionic liquid [Hmim]+BF4− was very convenient being obtained by simply mixing 1-methylimidazole with tetrafluoroboric acid (40% aq.) at 0–5 °C and stirring for 2 h at room temperature, followed by removal of water. The ionic liquid [Hmim]+BF4− was obtained in quantitative yield.Esterification of a variety of acids with four common alcohols was carried out in [Hmim]+BF4−. The results are listed in Table 1. Good to excellent yields and perfect selectivity were obtained in all cases. No by-products such as olefins were detected. And all of the esters produced could be easily separated due to their immiscibility with the ionic liquid. It is noteworthy that removal of water or using an excess of reactant is not necessary. No volatile organic solvents are required during the work-up and recycle of the ionic liquid [Hmim]+BF4−.
| Entry | Acid | Alcohol | Conversiona (%) | Selectivity to ester (%) | Time/h |
|---|---|---|---|---|---|
| a The conversion of acid or alcohol (ratio of acid to alcohol = 1∶1).b The esters are all double esterification products. No monoesterification product was detected; ratio of acid to alcohol = 1∶2. | |||||
| 1 | Acetic acid | 1-Butanol | 97 | 100 | 2 |
| 2 | Acetic acid | 1-Octanol | >99 | 100 | 2 |
| 3 | n-Decanoic acid | 1-Butanol | 96 | 100 | 3 |
| 4 | n-Decanoic acid | 1-Ocatanol | 97 | 100 | 3 |
| 5 | n-Decanoic acid | Methanol | 97 | 100 | 5 |
| 6 | Stearic acid | 1-Butanol | >99 | 100 | 3 |
| 7 | Stearic acid | 1-Octanol | >99 | 100 | 3 |
| 8 | Stearic acid | Methanol | >99 | 100 | 6 |
| 9 | Undecanoic acid | 1-Butanol | >99 | 100 | 3 |
| 10 | Undecylenic acid | 1-Butanol | >99 | 100 | 3 |
| 11 | Lactic acid | 1-Butanol | >99 | 100 | 2 |
| 12 | Crotonic acid | Methanol | 93 | 100 | 6 |
| 13 | Oxalic acid | Ethanol | >99 | 100b | 4 |
| 14 | Oxalic acid | 1-Octanol | >99 | 100b | 4 |
| 15 | Benzoic acid | 1-Butanol | 80 | 100 | 10 |
| 16 | 3-Hydroxybenzoic acid | 1-Butanol | 93 | 100 | 10 |
The results in Table 1 showed that the esterification of aliphatic acids with primary alcohols was very satisfactory, and the length of alkyl chains did not affect the conversion and the selectivity. Olefinic bonds of aliphatic acids were not changed under these reaction conditions (Table 1, entries 10 and 12). The hydroxy group of lactic acid did not take part in the esterification with only n-butyl lactate being obtained (Table 1, entry 11).
In the reaction of oxalic acid, no monoesterification products were detected when the molar ratio of acid to alcohols was 1∶2 (Table 1, entries 13 and 14).
Esterification of aromatic acids gave lower yields compared with those of aliphatic acids, further substituent groups on the aryl ring affected the reactions (Table 1, entries 15 and 16).
The esterification of methanol (Table 1, entries 5, 8 and 12) was not very easy due to its low boiling point and the required reaction time was longer than that of the other alcohols. However, satisfactory results were obtained.
The water produced in the reactions did not need to be removed because the ionic liquid [Hmim]+BF4− was miscible with water while the esters were immiscible with the ionic liquid [Hmim]+BF4−. Hence the esterification proceeded smoothly to completion. Liquid esters could be separated conveniently by decanting. Some esters (Table 1, entries 6–8, 15 and 16) have to be isolated above their melting point since they solidify at room temperature.
The ionic liquid [Hmim]+BF4− could be easily recycled. After reaction, with the mixture decanted, the ionic liquid [Hmim]+BF4− was separated and reused after drying in vacuum. The [Hmim]+BF4− was utilized repeatedly over eight times in the esterifacation of acetic acid with 1-butanol with the conversion and selectivity being unchanged (Table 2).
| Run | Conversion (%) | Selectivity to ester (%) | Time/h |
|---|---|---|---|
| 1 | 97 | 100 | 2 |
| 2 | 96 | 100 | 2 |
| 3 | 97 | 100 | 2 |
| 4 | 95 | 100 | 2 |
| 5 | 96 | 100 | 2 |
| 6 | 95 | 100 | 2 |
| 7 | 94 | 100 | 2 |
| 8 | 94 | 100 | 2 |
In summary, the esterification of carboxylic acid with alcohol, using the ionic liquid [Hmim]+BF4− as catalyst and solvent, has several advantages: (1) the ionic liquid [Hmim]+BF4−, as a strong Brønsted acid, shows superior catalytic activity than the systems reported by Fraga-Dubreuil et al.6 (2) The preparation of [Hmim]+BF4− was very simpe. (3) [Hmim]+BF4− could be directly reused after removal of water since no by-products were produced in the reaction. (4) The esters produced can be isolated conveniently in high yields and purity.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 20172016), Shanghai Phosphor Project of Science & Technology for Excellent Young Research (No. 01QA14017), the Foundation of Shanghai Science and Technology Development.References
- R. C. Larock, Comprehensive Organic Transformations, VCH, New York, 2nd edn., 1999 Search PubMed.
- M. Mäki-Arfela, T. Salmi, M. Sundell, K. Ekman, R. Peltonen and J. Lehtonen, Appl. Catal. A: Gen., 1999, 184, 25 CrossRef.
- M. Hino and K. Arata, Appl. Catal., 1985, 18, 401 CrossRef CAS.
- M. A. Schwegler and H. van Bekkum, Appl. Catal, 1999, 74, 191.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef.
- J. Fraga-Dubreuil, K. Bourahla and M. Rahmouni, Catal. Commun., 2002, 3, 185 Search PubMed.
- J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133–2139 RSC.
| This journal is © The Royal Society of Chemistry 2003 |
