Manganese air exposure assessment and biological monitoring in the manganese alloy production industry
Dag G. Ellingsen*, Siri M. Hetland and Yngvar Thomassen
National Institute of Occupational Health, P.O. Box 8149 Dep., N-0033, Oslo, Norway. E-mail: dag.ellingsen@stami.no; Fax: 47/23195205; Tel: 47/23195377
First published on 2nd December 2002
Abstract
One hundred workers carried personal air sampling equipment during three days to assess exposure to inhalable and respirable Mn. A novel four-step chemical fractionation procedure developed for the speciation of Mn in workroom aerosols was applied for selected aerosol filters. Blood and urine samples were analysed for Mn. The geometric mean (GM) concentrations of inhalable (n = 265) and respirable (n = 167) Mn determined in all filters were 254 µg m−3 and 28 µg m−3 respectively. Only 10.6% (95% CI 8.9–12.5) respirable Mn was found in the inhalable fraction when inhalable and respirable samples collected in parallel were considered (n = 153 pairs). There was a high correlation (Pearson's r = 0.70; p < 0.001) between respirable and inhalable Mn. The largest amounts of Mn in the inhalable aerosol fraction were found as Mn0 and Mn2+ (47.4%), whereas 28% was practically “insoluble”. The associations between B–Mn and aerosol concentrations of Mn were weak, but an association was found between U–Mn and respirable Mn; Pearson's r being 0.38 between “soluble” respirable Mn and U–Mn. No significant association was found between the “insoluble” components (probably SiMn) and Mn in biological samples.
Introduction
Manganese (Mn) compounds are well known neurotoxic substances which may cause manganism, a severe neurological disorder characterized by disturbances of movements.1,2 Occupational exposure to Mn may also induce impotency and behavioural symptoms such as hallucinations, bizarre behaviours and cognitive deficits.2,3 Manganese has several important functions in human physiology.4The widespread industrial use of Mn has drawn attention to its potential of causing severe neurological disease. Occupational exposure occurs during mining and crushing/milling of Mn ores and in the production of ferromanganese (FeMn) and silicomanganese (SiMn). The most important application of Mn is in the steel industry. Steel workers and welders are large groups of workers at risk of exposure. Other uses include for example Mn in dry cell batteries, in Mn-containing chemicals, and in non-ferrous alloys with aluminium.5 The use of the Mn-containing anti-knocking agent methylcyclopentadienyl-manganese tricarbonyl (MMT) as a fuel additive has received much attention in recent years.
Workers are exposed to different Mn species in the above-mentioned situations. Particle size distribution and solubility also vary considerably. Water soluble MnCl2 has mainly been used to gain toxicokinetic knowledge of Mn, whereas less soluble oxides and alloys are the most abundant forms of Mn occuring in the industry. Differences between the two compounds in the distribution pattern were shown in rats which were administered either MnCl2 or Mn3O4 intratracheally.6 The uptake of Mn was faster and the peak concentrations in blood were higher after the administration of MnCl2 than Mn3O4 or MnO2.6,7 The concentration of Mn in the cerebral target tissue striatum appears also to be higher after the instillation of MnCl2 compared to an equal amount of MnO2.7 Similarily, particle size distributions vary across industries. Welders, for instance, are exposed to relatively more Mn in the respirable aerosol fraction than Mn miners, who are exposed to coarser particles. Manganese may occur in all oxidation states between Mn3− and Mn7+, of which Mn2+ is chemically the most stable.8 The specific surface of MnO2 particles may be important, at least in respect to the inflammatory response of the lungs in mice.9 Differences in solubility, chemical composition, particle sizes and specific surfaces have received little attention in the literature, but are important in risk assessment.
Little information is available on the association between work room air exposure to Mn assessed by personal sampling and the concentration of Mn in urine (U-Mn) or blood (B-Mn). No association on an individual basis was found between the concentration of Mn in the work room air and the individual concentrations of B-Mn or U-Mn in Mn oxide and salt producing workers or dry alkaline battery workers.10,11 Exposure studies in Mn alloy-producing plants have, however, suggested an association between cumulative exposure and B-Mn,12 air levels of Mn and B-Mn,13 and airborne Mn and B-Mn and U-Mn respectively.14
This work is part of a larger epidemiological study of the potential health effects related to Mn exposure in Norwegian Mn alloy-producing plants. In general, Mn-alloy producing plants may be divided into producers of FeMn only, SiMn only, or producers of both alloys. Several Mn compounds occur during different stages of Mn alloy production, such as oxides, Mn metal, and Mn-alloys.8 This study aims to describe the exposure with respect to Mn in the respirable and inhalable aerosol fractions. Further, for the first time the chemical solubility of Mn compounds in the work room air collected as personal samples was assessed, and related to the individual concentrations of B-Mn and U-Mn.
Material and methods
Manganese alloy-producing plants
The three studied plants in Norway producing FeMn and/or SiMn employ approximately 700 workers. Two of them produce both FeMn and SiMn while the third produces SiMn only. The two FeMn producing plants also refine the FeMn with oxygen. In FeMn production, the charge consists of manganese lump ore, iron ore, coal and coke as reductants and limestone as a flux. The slag formed during FeMn production is reused for producing SiMn, where it is smelted with quartzite using coke and coal as the reducing agents and dolomite as a flux. The alloys are produced by heating the raw materials together in a furnace at temperatures from 500 to 1500 °C. Electricity is used to provide the required heat.8Study design and subjects
The data for biological and environmental monitoring reported in this paper were collected for the characterization of ongoing exposure as background data for an epidemiological study in the Norwegian Mn alloy producing industry. Only men who had been exposed to Mn for at least one year in a contaminated area of the Mn alloy-producing plants under study were eligible for inclusion in that cross-sectional study. The study included 100 Mn-exposed subjects, which were randomly selected among all employees in the production and maintenance in the three plants under scrutiny. As 10 subjects declined participation in the study, 110 subjects were invited (participation rate 90.9%). A corresponding number of age matched referents were selected from one plant producing silicon metal and one plant producing titanium dioxide and pig iron.All clinical examinations, including a structured interview, blood and urine sampling were carried out at the local occupational health clinics. Some background characteristics are shown in Table 1. Informed written consent was obtained from all participants. The study protocol was approved by the local ethical committee for medical research.
| Mean | Prevalence | Range | |
|---|---|---|---|
| Age (years) | 44.2 | — | 27.6–61.9 |
| Years exposed to Mn | 20.2 | — | 2.1–41.0 |
| Current smokers (%) | — | 50 | — |
| Shift workers (%) | — | 42 | — |
| Maintenance workers (%) | — | 32 | — |
| Production workers (%) | — | 68 | — |
| -Raw Material Department | — | 25 | — |
| -Furnace House | — | 33 | — |
| -End Product Department | — | 7 | — |
| -Others | — | 3 | — |
Air sampling strategy
The ongoing individual exposure of each worker to airborne Mn was characterised by personal full shift sampling. The sampling period was three days closely before and/or after the day of the blood and urine sample collection. Parallel sampling of inhalable and respirable aerosol fractions was carried out at two of the plants. At the third plant, inhalable sampling was carried out on the whole participating group, while a reduced number of parallel respirable samples were collected. Limited sampling of inhalable aerosol in the two reference plants was carried out in order to verify that the exposure to Mn was negligible. One reference plant produces silicon metal, whereas the other is a major producer of titanium dioxide/pig iron. The inhalable fraction is the mass fraction of total airborne aerosol that may be inhaled through the nose and mouth. The respirable fraction is the mass fraction of inhaled particles penetrating to the alveolar region.15Air sampling
The inhalable aerosol fraction was collected with IOM personal inhalable aerosol samplers (SKC Inc., Pennsylvania, USA). The respirable aerosol fraction was collected using Casella cyclones (Casella, London, England). The air flows through the filters were 2 and 2.2 l min−1, respectively. The filters used were membrane 0.8 µm cellulose ester type (AAWP02500 or AAWP03700, Millipore Corp., Bedford, MA, USA). Pulsation free sampling pumps PS-101, developed and produced at National Institute of Occupational Health (NIOH, Oslo, Norway), were used. An in-house calibrated rotameter (type V100-80.12, Vögtlin, Basel, Switzerland) was used to measure the flow rate of each pump at the beginning and at the end of the sampling period as a quality control measure.Chemical speciation/sequential extraction and measurements of total Mn in air filters
A novel four-step chemical fractionation procedure developed to characterise workroom aerosols in Mn alloy-producing plants was applied for the speciation of Mn in a selection of the collected air filters.16 The following components were quantified;Components 1: "Water soluble" Mn dissolved in 0.01 M ammonium acetate.
Components 2: Mn0 and Mn2+ dissolved in 25% acetic acid (Mn metal, FeMn, MnO and the Mn2+ part of the mixed oxide Mn3O4).
Components 3: Mn3+ and Mn4+ dissolved in 0.5% hydroxylamine hydrochloride in 25% acetic acid. (The non-dissolved Mn3+ part of Mn3O4, Mn2O3 and MnO2.)
Components 4: "Insoluble" Mn digested in aqua regia/hydrofluoric acid (SiMn).
Components 4 were only soluble in strong acids in the presence of hydrofluoric acid and were therefore defined as “insoluble” in this study. The other three components were more easily chemically soluble, and Components 1 + 2 + 3 were therefore defined as “soluble”.
Air filters for bulk elemental measurements were dissolved in a mixture of aqua regia/hydro-fluoric acid in Teflon autoclaves with microwave-assisted digestion. A Perkin-Elmer Optima Model 3000 inductively coupled plasma atomic emission spectrometer (Perkin-Elmer, Nor-walk, CT, USA) was used for all measurements.
In house, commercially available, reference filter material simulating workroom air concentrations at occupational exposure limit values for Mn (batches A-2 and B-2) and a mixture with identical weight ratios of FeMn, MnO, MnO2, Mn2O3, Mn3O4 and SiMn were used to monitor the accuracy and reproducibility of the measurements throughout the study. The measured values for total Mn were in excellent agreement with the certified value (≤2%) and the day-to-day variation was ≤1.5%.16
During the fractionation and instrumental measurement period, a total of 18 sub-samples of the quality control mixture were analysed. The recoveries of Mn in % ± SD for Components 1, Components 2, Components 3 and Components 4 were 107 ± 4, 92 ± 4, 107 ± 5 and 94 ± 3 respectively.16
Measurements of trace elements in urine and whole blood
The subjects were instructed to bring a first-voided morning urine from the day of the clinical examinations in 25 ml NUNC® containers (Nalge Nunc International, Denmark). The samples were voided at home. The containers were put into transport containers and finally into small closed plastic bags to avoid contamination. As the urines were voided at home, no contact was possible between the working clothes and the urine samples. The urine samples were stored at −20 °C in refrigerators at the respective occupational health clinics until transport to NIOH for analysis.Heparinized whole blood was collected between 08.00 and 09.00 on the same day with 10 ml Venoject® (Terumo Corp., Belgium) tubes from the cubital vein. The samples were stored at −20 °C until analysis. The Mn contamination from the sampling equipment used throughout the study was tested by drawing a 0.5% nitric acid aqueous solution into 5 randomly selected blood tubes with needles or filling the NUNC containers completely with the leaching solution. After keeping the tubes/containers at room temperature for 24 h, Mn concentrations were measured in the leachates to be 0.5 ±0.2 (blood) and <0.02 (urine) µg L−1, respectively. These concentrations were not considered to contribute to the analytical results and thus no corrections have been made to the reported Mn concentrations.
Manganese (U-Mn) in urine
U-Mn was measured after dilution to 1∶1 with 0.5% nitric acid by electrothermal atomic absorption spectrophotometry (EAAS) using a Perkin-Elmer SIMAA 6000 system calibrated with urine-matched standard solutions.Manganese (B-Mn) and lead (B-Pb) in whole blood
For the measurement of B-Mn and B-Pb, 2.5 ml of ultrapure 65% nitric acid was added to 2 ml of whole blood in a polypropylene digestion tube. The tube was heated to 95 °C for 1 h and after cooling the sample was diluted to volume (13.7 ml). The solution was analysed simultaneously for Mn and Pb by EAAS (Perkin-Elmer SIMAA 6000).Accuracy and reproducibility of trace element measurements
Accuracy and reproducibility were assessed using Seronorm human urine (batch 4043125) and whole blood (batches 404107 and 404108) reference materials (Sero Ltd, Asker, Norway). U-Mn and B-Mn were measured in these materials with a day-to-day variation of 6.5%, 3.5% (in 404107) and 5.0% (in 404108) respectively. The results agreed well with the values recommended by the producer; U-Mn: recommended 13 µg l−1 , measured 10.8 (SD 0.7) µg l−1 (N = 15); B-Mn in 404107: recommended 9 µg l−1; measured 8.2 (SD 0.3) µg l−1 (N = 21); B-Mn in 404108; recommended 13 µg l−1; measured 12.0 (SD 0.6) µg l−1 (N = 22). The reference materials results for B-Pb was also acceptable showing typically day-to-day variations ≤7% and accuracies within ± 10%.Statistics
Most of the measured variables reported had skewed distribution by visual inspection. The variables were log-transformed if the skewness exceeded 2.0, in order to allow the use of parametric statistical analysis. Analysis of variance was used for group comparisons of continuous variables. If more than two groups were compared, the least square difference was calculated posthoc in order to separate the groups that differed. Correlations between independent variables were assessed with Pearson's least square regression analysis, yielding Pearson's correlation coefficients. The level of statistical significance was set two-tailed at p < 0.05. The statistical package SPSS®, version 8.0, was used for statistical calculations.Results
Table 2 shows the concentrations of Mn in the inhalable and the respirable aerosol fractions measured in all collected personal air filters. The geometric mean (GM) concentration of inhalable Mn (254 µg m−3) was approximately 10-fold higher than the GM concentration of respirable Mn (28 µg m−3). For comparison, a GM of 4 µg Mn m−3 (95% CI 3–5; n = 58) in the inhalable aerosol fraction was found in the reference plants. The speciation of Mn in the inhalable and respirable aerosol fractions by chemical leaching showed that little Mn was present as “water soluble” Components 1. The highest amounts of Mn were found as Components 2 for both the respirable and the inhalable aerosol fractions, followed by the “insoluble” Components 4.| AMa | GMb | Min | Max | Percentile | |||
|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | |||||
| a AM: arithmetic mean.b GM: geometric mean. | |||||||
| Inhalable (n = 265) | 769 | 254 | 7 | 27200 | 91 | 245 | 624 |
| Respirable (n = 167) | 64 | 28 | 1 | 1011 | 10 | 29 | 68 |
| Chemical leaching of inhalable Mn (n = 199): | |||||||
| Components 1 | 26 | 14 | 1 | 350 | 6 | 15 | 30 |
| Components 2 | 367 | 113 | 4 | 14000 | 39 | 113 | 306 |
| Components 3 | 216 | 31 | <0.5 | 9360 | 9 | 29 | 101 |
| Components 4 | 183 | 49 | 0.5 | 5990 | 17 | 51 | 122 |
| all | 791 | 258 | 10 | 27200 | 93 | 249 | 632 |
| Chemical leaching of respirable Mn (n = 150): | |||||||
| Components 1 | 3 | 2 | <0.5 | 21 | 1 | 3 | 4 |
| Components 2 | 33 | 13 | <0.5 | 693 | 5 | 14 | 35 |
| Components 3 | 12 | 2 | <0.5 | 201 | 1 | 2 | 8 |
| Components 4 | 11 | 4 | <0.5 | 126 | 2 | 4 | 10 |
| All | 60 | 26 | 1 | 1011 | 9 | 28 | 67 |
Table 3 shows that the proportions of Mn in the respirable aerosol fraction related to Mn in the inhalable aerosol fraction are quite similar among workers in different departments or groups of workers. However, workers in the furnace house had on average somewhat higher proportions of respirable manganese, which was mainly related to the substantially higher proportion among the crane operators (39.1%). A proportion of 10.6% was calculated when all inhalable and respirable samples collected in parallel (n = 153 pairs) were considered. The Pearson's correlation coefficient (r = 0.70; p < 0.001, n = 153) calculated between the concentration of Mn in the respirable fraction and in the inhalable aerosol fraction was high (Fig. 1).
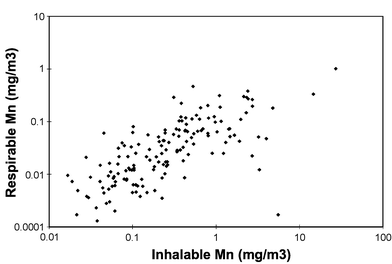 | ||
| Fig. 1 The association between Mn in the respirable and the inhalable aerosol fractions (n = 153; Pearson's r = 0.70; p < 0.001). | ||
| N | GMa (%) | 95% CIb | |
|---|---|---|---|
| a GM; geometric mean.b 95% CI; 95% confidence interval. | |||
| All samples | 153 | 10.6 | 8.9-12.5 |
| Raw Material Department | 42 | 8.8 | 6.0-12.9 |
| Furnace House/smelting operations | 43 | 14.3 | 10.8-18.9 |
| -Furnace workers | 14 | 15.6 | 11.5-21.3 |
| -Crane operators | 9 | 39.1 | 27.5-55.5 |
| Manufactured Product Department | 11 | 7.4 | 3.8-14.5, |
| Mechanics/electricians | 45 | 11.5 | 8.4-15.7 |
Inhalable air samples were collected from 71 workers on three different days. The variability of exposure between days was studied for these subjects by calculating the coefficient of variation (CV) for each individual. The mean CV was 62.3% (95% CI 53.4–71.2). Similarly a CV of 66.8% (95% CI 52.4–81.3) was obtained for personal respirable samples which were collected from 49 subjects on three different days. Data sets of both respirable and inhalable samples collected on three different days were available for 38 workers. The mean CV for the measured concentrations of airborne Mn was 69.0% (95% CI 55.8–82.1) and 72.3% (95% CI 55.6–88.9) for the inhalable fraction and the respirable aerosol fraction respectively. The GM concentrations of Mn in the inhalable aerosol fraction were quite similar for the three days of air sample collection for the 71 workers with complete data sets (day 1: GM 267 µg m−3, 95% CI 191–374; day 2: GM 250 µg m−3, 95% CI 178–352; day 3: GM 263 µg m−3, 95% CI 188–368). The corresponding concentrations of respirable Mn measured for the 49 workers with complete data sets showed greater variations; day 1: GM 27 µg m−3, 95% CI 18–39; day 2: GM 21 µg m−3, 95% CI 14–32; day 3: GM 33 µg m−3, 95% CI 23–47.
Table 4 shows the percentages of Mn present as different components according to the chemical leaching in relation to the total concentrations of Mn found in the air filters. Overall, a GM of 8.2% of Mn found in the inhalable aerosol fraction was “water soluble” (Components 1), and 28.0% was practically “insoluble” (Components 4). The percentage of “insoluble” Mn (Components 4) was substantially higher in the plant producing only SiMn (48.1%) than in the two plants producing both FeMn and SiMn (17.9%) (p < 0.001). Manganese in the respirable aerosol fraction was somewhat more soluble, and a lower percentage of Mn was found as “insoluble” Components 4 compared to the inhalable aerosol fraction.
| Components 1 | Components 2 | Components 3 | Components 4 | |||||
|---|---|---|---|---|---|---|---|---|
| AMa | 95% CI | AM | 95% CI | AM | 95% CI | AM | 95% CI | |
| a AM: arithmetic mean.b All = all available filters.c SiMn = all available filters from the plant producing only SiMn.d SiMn/FeMn = all available filters from plants producing SiMn and FeMn. | ||||||||
| Inhalable Mn | ||||||||
| Allb (n = 199) | 8.2 | 7.1–9.2 | 47.4 | 45.3–49.5 | 16.4 | 14.5–18.3 | 28.0 | 24.9-31.1 |
| SiMnc (n = 67) | 5.0 | 3.3–6.6 | 37.9 | 33.7–42.1 | 9.0 | 7.7–10.4 | 48.1 | 42.7-53.5 |
| SiMn/FeMnd (n = 132) | 9.8 | 8.5–11.1 | 52.2 | 50.3-54.2 | 20.1 | 17.6–22.6 | 17.9 | 15.5-20.2 |
| Respirable Mn | ||||||||
| All (n = 150) | 10.8 | 9.6–12.1 | 53.7 | 51.0–56.4 | 12.1 | 10.5–13.6 | 23.4 | 20.3-26.5 |
| SiMn (n = 73) | 11.2 | 9.5–12.9 | 47.2 | 43.2–51.1 | 9.3 | 8.1–10.5 | 32.3 | 28.2-36.4 |
| SiMn/FeMn (n = 77) | 10.5 | 8.8–12.3 | 59.8 | 56.7–63.0 | 14.6 | 11.9–17.4 | 15.0 | 11.2-18.7 |
Manganese Components 1–4 measured in the respirable aerosol fraction were compared to the same Mn components of the inhalable aerosol fraction in filters which had been sampled in parallel (n = 138 pairs of filters). A GM of 19.0% (95% CI 16.1–22.4) of Mn in Components 1 in the inhalable aerosol fraction was respirable. Correspondingly, the figures for Components 2, 3, and 4 were respectively; GM 12.5% (95% CI 10.3–15.0), GM 9.5% (95% CI 7.6–12.0), and GM 5.3% (95% CI 4.2–6.7) (not tabulated).
The concentrations of B-Mn, U-Mn and B-Pb were statistically significantly higher in the Mn-exposed workers compared to the referents (Table 5). The available air concentrations for each individual were used to calculate an average inhalable “manganese exposure dose” at the time of the examinations. The results are shown in Table 5. These individual dose estimates were further used to assess associations between the concentrations of Mn in biological samples and the concentrations of Mn in the work room air.
| Exposed | Referents | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AMa | GMb | Min | Max | AM | GM | Min | Max | ||
| a AM: arithmetic mean.b GM: geometric mean.c n = 59.d n = 97.e n = 53.f Soluble: Components 1 + 2 + 3.g cr.: creatinine. | |||||||||
| B-Mn/nmol l−1 | 189 | 181 | 84 | 426 | 166 | 160 | 72 | 374 | 0.002 |
| U-Mn /nmol mmol (cr.)−1g | 3.9 | 0.9 | 0.1 | 126.3 | 0.9 | 0.4 | 0.1 | 13.1 | <0.001 |
| B-Pb/µmol l−1 | 0.20 | 0.16 | 0.01 | 1.27 | 0.15 | 0.12 | 0.01 | 0.58 | 0.001 |
| Mninhalable/µg m−3 | 753 | 301 | 9 | 11457 | — | — | — | — | — |
| Mnrespirable /µg m−3)c | 64 | 36 | 3 | 356 | — | — | — | — | — |
| Mninhalable/solublef/µg m−3d | 570 | 197 | 9 | 9001 | — | — | — | — | — |
| Mnrespirable/soluble/µg m−3e | 49 | 25 | 2 | 320 | — | — | — | — | — |
Table 6 shows the Pearson's correlation coefficients between the individual “manganese exposure dose” in the inhalable and respirable aerosol fractions and the concentrations of U-Mn and B-Mn respectively. There were no statistically significant associations between B-Mn and the total concentrations of Mn in the inhalable aerosol fraction, nor any of the components of the inhalable aerosol, based on the measurements of the chemical leaching. The correlation coefficients were somewhat higher when the associations to U-Mn were assessed. Statistically significantly weak associations were observed between U-Mn and respectively, total and “soluble” Mn in the inhalable aerosol fractions. Generally, higher correlation coefficients were calculated between Mn in the respirable aerosol fraction and the biological indices of exposure (Fig. 2). The best fit was found for the association between U-Mn and Components 3 in the respirable aerosol fraction (Pearson's r = 0.48; p <0.001) . It is noteworthy that the association between chemically “insoluble” Mn (Components 4) in the respirable aerosol fraction and U-Mn did not attain statistical significance.
 | ||
| Fig. 2 The association between U-Mn and Mn found in “soluble” components (1 + 2 + 3) of the respirable aerosol fraction (n = 53; Pearson's r = 0.38; p <0.01). | ||
| N | B-Mn | U-Mn (log) | |
|---|---|---|---|
| a p < 0.05.b p < 0.01.c p < 0.001. | |||
| Inhalable Mn: | |||
| Components 1 (log) | 97 | 0.12 | 0.09 |
| Components 2 (log) | 97 | 0.09 | 0.22a |
| Components 3 (log) | 97 | 0.09 | 0.20 |
| Components 4 (log) | 97 | 0.01 | 0.17 |
| All components (log) | 100 | 0.15 | 0.23a |
| “Soluble” Components (1 + 2 + 3) (log) | 97 | 0.09 | 0.21a |
| Respirable Mn: | |||
| Components 1 (log) | 53 | 0.22 | 0.26 |
| Components 2 (log) | 53 | 0.24 | 0.35a |
| Components 3 (log) | 53 | 0.27a | 0.48c |
| Components 4 (log) | 53 | 0.22 | 0.24 |
| All components (log) | 59 | 0.24 | 0.34b |
| “Soluble” Components (1 + 2 + 3) (log) | 53 | 0.25 | 0.38b |
To further elucidate the associations between air and biological measures of exposure, the exposed subjects with “high” concentrations of B-Mn or U-Mn were compared with the exposed subjects with “low” concentrations of B-Mn or U-Mn (Table 7). Subjects with “high” B-Mn had higher B-Pb, and subjects with “high” U-Mn had higher air exposure measures than those with “low” B-Mn or U-Mn.
| B-Mn | U-Mn | |||
|---|---|---|---|---|
| “High” (n = 11) | “Low” (n = 89) | “High” (n = 15) | “Low” (n = 85) | |
| Years exposed | 21.8 | 19.8 | 20.3 | 20.0 |
| Inhalable Mn/µg m−3 | 351 | 296 | 537 | 272p = 0.07 |
| Inhalable Mnsoluble/µg m−3 | 201 | 197 | 325 | 180p = 0.14 |
| Respirable Mn/µg m−3 | 49 | 35 | 70 | 31p = 0.030 |
| Respirable Mnsoluble/µg m−3 | 35 | 24 | 57 | 20p = 0.008 |
| B-Pb/µmol l−1 | 0.23 | 0.16p = 0.025 | 0.16 | 0.16 |
| U-Hg/nmol mmol (cr.)−1 | 1.0 | 1.0 | 0.9 | 1.1 |
| Current smoking/g week−1 | 36 | 41 | 44 | 40 |
Discussion
The study describes some characteristics of exposure to Mn in Mn alloy producing plants, and associations between Mn in the work room air and Mn measured in biological samples of exposed workers. Acknowledging that exposure to Mn in this industry is a complex issue, a selection of the air filters which had been collected were objects of a novel chemical leaching procedure for the characterisation of the collected Mn-containing aerosols.16The GM concentration of Mn in the inhalable aerosol fraction of the exposed workers was 301 µg m−3, of which 10.6% was found in the respirable aerosol fraction. This suggests that a low percentage of the inhaled Mn-amount penetrates into the deeper parts of their lungs. Thus, most of the Mn may end up in the gastrointestinal tract, where even the water soluble MnCl2 is taken up to a limited extent.17 Mn-containing particles from the work room air of Mn alloy-producing plants are likely to be less soluble than MnCl2, and lower gastrointestinal uptake of MnO2 compared to MnCl2 has been shown.7
Surprisingly few studies have focused on the important issue of particle size distribution of work room air particulates in the Mn-alloy producing industry. One study, however using stationary parallel sampling, reported a share of Mn in the respirable aerosol fraction comparable to our results.18 The few other studies have reported that 40–60% of the Mn is found in the respirable aerosol fraction,12,13 or a ratio from 2.0 to 2.8 between total particulate Mn and Mn in the respirable aerosol fraction.14 These latter studies used air samplers for the collection of “total” aerosol. Such samplers underestimate the inhalable aerosol fraction to a large extent, often with a factor of 2–3 for coarse dust (extrathoracic). It could therefore be that the “total” Mn exposure measured in those studies is much lower than if the “inhalable fraction” had been assessed,19 resulting in a higher percentage of respirable Mn in relation to the “total” dust. Knowledge of the particle size distribution is fundamental for appropriate risk assessment, in particular for Mn because of the low gastrointestinal uptake, and also because of the local effects on the lungs. As the estimates of the magnitude of the respirable aerosol fraction are quite diverging, it is of importance to obtain more information about the distribution of health-related aerosol fractions in this industry.
The exposure to Mn varied substantially between days; the mean CV's for Mn in the inhalable and respirable aerosol fractions being between 60 and 70%. Air samples should therefore be collected over several days in order to establish reliable individual estimates of ongoing exposure in this industry. It is noteworthy that the 49 workers where respirable samples were collected on three days on average had nearly 60% higher exposure to Mn in the respirable aerosol fraction on day 3 compared to day 2. This knowledge also calls for caution when calculating socalled cumulative exposure estimates in epidemiological research. We have not found any other reports on the variability of individual exposure to Mn in this industry.
Some results from the chemical leaching applied to speciate Mn deserve attention. Only 8.2% of the amount of Mn found in the inhalable aerosol fraction was “water soluble” (Components 1), and 28.0% was practically “insoluble” (Components 4), i.e. soluble only in a mixture of aqua regia/hydrofluoric acid or hydrofluoric/nitric acids. We assume that the bioavailability of Components 4 is low. The plant producing only SiMn had a substantially higher percentage of Components 4 than the plants producing both FeMn and SiMn in the inhalable aerosol fraction (48.1% vs. 17.9%). Experimental data suggest that SiMn is the main Mn species in the “insoluble” Components 4.16 An implication of this result could be that the production of SiMn may result in relatively lower exposure to more easily bioavailable other Mn-compounds. This point has received no attention in the literature, but could have practical consequences for the Mn alloy producing industry. It is likely that plants producing only SiMn have a higher share of SiMn in their work environment than producers of both alloys as two of the plants in this study. It is known from the literature that exposure of rats to water soluble Mn compounds (MnSO4, MnCl2) results in higher concentrations of Mn in the critical area of effect in the brain, striatum, than exposure to the less soluble Mn3O4 or MnO2.7,20 The pulmonary and gastrointestinal uptake of SiMn and resulting brain concentrations should be studied, because a potentially low uptake of SiMn may have important implications for risk assessment in this industry. Exposure to Mn in the Italian Mn alloy-producing industry has been stated to consist of around 95% Mn oxides or MnO2 and Mn3O4.12,13 Our results do not support this conclusion, and exposures in Mn alloy-producing plants appear to be more complex than previously reported. The highest concentration of Mn was found in Components 2, suggesting Mn metal, FeMn, MnO, and Mn2+ from Mn3O4 to be the most abundant Mn species.
The concentration of B-Mn was about 15% higher, and U-Mn about twice as high in the exposed group compared to the referents. The concentration differences between exposed and referents are lower than in other studies of Mn-exposed workers10,11,13,18,21,22 with one23 exception. This could indicate that the exposure to bioavailable Mn is comparatively low in this group of workers. The interpretation of B-Mn may be difficult, because “normal” concentrations in unexposed referents appear to differ quite substantially across the aboved mentioned studies, i.e. from mean 5.7 µg l−110,13 to mean 23.3 µg l−123 (103 nmol l−1 to 419 nmol l−1). In studies of healthy Italians or Americans, mean B-Mn concentrations of 8.8 µg l−1 (158 nmol l−1) and 10.9 µg l−1 (198 nmol l−1) were reported.24,25 It should be further elucidated if these large differences in B-Mn concentrations are related to sampling or measurement errors, or really reflect regional differences. Without further knowledge on this subject, the use of B-Mn in the assessment of Mn toxicity should in our opinion be made with caution.
B-Mn in the exposed workers was not associated with the individual concentrations of Mn in the inhalable aerosol fraction, but a weak association with Mn in the respirable aerosol fraction cannot be excluded. Two studies have reported associations between Mn in “total” dust and B-Mn,13,14 whereas other studies have not confirmed an association.10,11 The exposed workers in this study with the highest B-Mn did not differ from the remaining exposed subjects with respect to their current exposure to airborne Mn (Table 7). However, their higher B-Pb can of course be a result of co-exposure of Mn and Pb in these workers. Interestingly, several studies have suggested associations between the concentration of B-Mn and B-Pb in populations apparently not occupationally exposed to Mn.26,27,28 Increased protoporphyrin in erythrocytes induced by lead exposure, resulting in increased uptake of Mn into the erythrocytes, has been suggested as a mechanism for this possible association.29
We found weak associations between U-Mn and the individual amounts of Mn in the inhalable aerosol fractions. However, the correlation coefficients were substantially higher when U-Mn was related to Mn in the respirable aerosol fraction. The data also show a higher correlation between U-Mn and “soluble” respirable Mn (Components 1 + 2 + 3), than Mn in the respirable aerosol fraction (Components 1 + 2 + 3 + 4). The amount of “insoluble” Mn (Components 4) was not statistically significantly associated with U-Mn. The results could suggest that “soluble” Mn in the respirable aerosol fraction is the most appropriate predictor for exposure to bioavailable Mn compounds in Mn alloy-producing plants, and further that SiMn may be of less importance with respect to the total Mn uptake. The few published studies on the association between U-Mn and Mn in the work room air have shown conflicting results. Apostoli etal.14 reported a statistically significant association between U-Mn and Mn in the work room air on an individual basis in Mn alloys workers. Studies of alkaline battery plant workers exposed to manganese dioxide and workers from a Mn oxide and salt-producing plant did not reveal an association.10,11
In summary, only 10.6% respirable Mn was found in the inhalable aerosol. A novel four-step chemical fractionation procedure developed for the speciation of Mn in workroom aerosols suggested that the largest amounts of Mn in the inhalable aerosol fraction were found as Mn0 and Mn2+ (47.4%), whereas 28% was practically chemically “insoluble” SiMn. The strongest association between air measures of exposure and biological samples was found between U-Mn and “soluble” respirable Mn. This could suggest that the “soluble” compounds in the respirable aerosol fraction may be the most relevant with respect to uptake into the body. Whether SiMn has a lower bioavailability and perhaps a lower potential for inducing manganese related systemic health effects than the other detected Mn compounds, remains to be elucidated.
Acknowledgements
The skilful laboratory work of Gunhild Sand and Elaine Nielsen at the National Institute of Occupational Health is appreciated. Erle Grieg Astrup, ELKEM ASA, is thanked for her contribution during the planning stage of this study. The study was financially supported by the Work Environment Fund of the Norwegian Business Association, Elkem ASA, Norway, Tinfos Jernverk AS, Norway and ERAMET ASA Norway.References
- J. Couper, Br. Ann. Med. Pharm., 1837, 1, 41 Search PubMed.
- D. E. McMillan, Neurotoxicology, 1999, 20, 499 CAS.
- J. Rodier, Br. J. Ind. Med., 1955, 12, 21 Search PubMed.
- F. C. Wedler, in Biological significance of manganese in mammalian systems, Progress in Medical Chemistry, eds. G. P. Elvers and D. K. Luscombe, Elsevier Medical Publishers BV, Amsterdam, 1993, p. 89 Search PubMed.
- A. H. Reidies, in Manganese compounds, Ullman's Encyclopedia of Industrial Chemistry, eds. B. Elvers, S. Hawkins and G. Schulz, VCH Verlagsgesellschaft mbH, Weinheim, 5th edn., 1990, vol. A16, p. 123 Search PubMed.
- D. B. Drown, S. G. Oberg and R. P. Sharma, J. Toxicol. Environ. Health, 1986, 17, 201 CAS.
- H. Roels, G. Meiers, M. Delos, I. Ortega, R Lauwerys, J. P. Buchet and D. Lison, Arch. Toxicol., 1997, 71, 223 CrossRef CAS.
- D. B. Wellbeloved, P. M. Craven and J. W. Waudby, in Manganese and manganese alloys, Ullman's Encyclopedia of Industrial Chemistry, eds. B. Elvers, S. Hawkins and G. Schulz, VCH Verlagsgesellschaft mbH, Weinheim, 5th edn., 1990, vol. A16, p. 77 Search PubMed.
- D. Lison, C. Lardot, F. Huaux, G. Zanetti and B Fubini, Arch. Toxicol., 1997, 71, 725 CrossRef CAS.
- H. Roels, R. Lauwerys, P. Genet, M. S. Sarhan, M. De Fays, I. Hanotiau and J. P. Buchet, Am. J. Ind. Med., 1987, 11, 297 CAS.
- H. A. Roels, P. Ghyselen, J. P. Buchet and R. R. Lauwerys, Br. J. Ind. Med., 1992, 49, 25 Search PubMed.
- R. Lucchini, L. Selis, D. Folli, P. Apostoli, A. Mutti, O. Vanoni, A. Iregren and L. Alessio, Scand. J. Work. Environ. Health, 1995, 21, 143 Search PubMed.
- R. Lucchini, P. Apostoli, C. Perrone, D. Placidi, E. Albini, P. Migliorati, D. Mergler, M. P. Sassine, S. Palmi and L. Alessio, Neurotoxicology, 1999, 20, 287 CAS.
- P. Apostoli, R. Lucchini and L. Alessio, Am. J. Ind. Med., 2000, 37, 283 CrossRef CAS.
- European Standard EN 481, Workplace atmospheres–size fractionation definitions for 15 measurements of airborne particles, European Committee for Standardization, Bruxelles, 16 Belgium, 1993 Search PubMed.
- Y. Thomassen, D. G. Ellingsen, S. Hetland and G. Sand, J. Environ. Monit., 2001, 3, 555 RSC.
- G. Oberdoerster and G. Cherian, in Manganese, Biological Monitoring of Toxic Metals, eds. T. W. Clarkson, L. Friberg, G. F Nordberg and P. R. Sager, Plenum Press, New York, NY, 1988, p. 283 Search PubMed.
- D. Mergler, G. Huel, R. Bowler, A. Iregren, S. Belanger, M. Baldwin, R. Tardif, A. Smargiassi and L. Martin, Environ. Res., 1994, 64, 151 CrossRef CAS.
- J. H. Vincent, in Aerosol Sampling, Science and Practice, John Wiley & Sons, New York, NY, 1989 Search PubMed.
- D. C. Dorman, M. F. Struve, R. Arden James, M. W. Marshall, C. U. Parkinson and B. A. Wong, Toxicol. Appl. Pharmacol., 2001, 170, 79 CrossRef CAS.
- J. D. Wang, C. C. Huang, Y. H. Hwang, J. R. Chiang, J. M. Lin and J. S. Chen, Br. J. Ind. Med., 1989, 46, 856 Search PubMed.
- R. Lucchini, E. Bergamaschi, A. Smargiassi, D. Festa and P. Apostioli, Environ. Res., 1997, 73, 175 CrossRef CAS.
- S. E. Chia, S. C. Foo, S. L. Gan, J. Jeyaratnam and C. S. Tian, Scand. J. Work. Environ. Health, 1993, 19, 264 Search PubMed.
- D. B. Milne, R. L. Sims and N. V. C. Ralston, Clin. Chem., 1990, 36, 450 CAS.
- C. Minoia, E. Sabbioni, P. Apostoli, R. Pietra, L. Pozzoli, M. Gallorini, G. Nicolaou, L. Alessio and E. Capodaglio, Sci. Total Environ., 1990, 95, 89 CrossRef CAS.
- R. L. Zielhuis, P. del Castilho, R. F. M. Herber and A. A. E. Wibowo, Environ. Health Perspectives, 1978, 25, 103 Search PubMed.
- M. M. Joselow, E. Tobias, R. Koehler, S. Coleman, J. Bogden and D. Gause, Am. J. Public Health, 1978, 68, 557 Search PubMed.
- J. Saavedra-Contreras and C. Rios, Arch. Invest. Med., 1991, 22, 57 Search PubMed.
- A. A. E. Wibowo, H. J. A. Sallè, P. del Castilho and R. L. Zielhuis, Int. Arch. Occup. Environ. Health, 1979, 43, 177 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
