Measurements of C6–C8 hydrocarbons at a UK rural site during January 1999. Site evaluation and correlations between species
J. R.
Hopkins†
,
C. J.
Barnett
,
A. C.
Lewis†
and
P. W.
Seakins
*
Department of Chemistry, University of Leeds, Leeds, UK LS2 9JT
First published on 31st October 2002
Abstract
Ambient concentrations of C6–C8 aromatic hydrocarbons and n-heptane, determined by gas chromatography with flame ionisation detection, are presented from a winter campaign during January 1999 at a rural site near Leeds. Absolute concentrations are significantly lower than those obtained from the only designated UK rural site (Harwell) in the automated UK hydrocarbon network. Both absolute and relative concentrations of hydrocarbons measured at the site have been interpreted in terms of the arriving back-trajectories. The site is subject to two main airflows during the winter months; relatively polluted air from the southwest and much cleaner air from the northwest. Ratios of hydrocarbon concentrations show evidence of significant chemical processing consistent with chemical removal by OH. Uncertainties in the ages of the trajectories prevent a reliable estimation of the average OH concentration over the trajectory. The dependence of the variance of the hydrocarbon concentrations with their lifetime with respect to removal by OH does not show the expected behaviour.
Introduction
Many extensive measurements exist on urban hydrocarbon concentrations. In the UK, the operation of the Automated Urban Network (AUN) has provided a wealth of data.1 However, much less is known about long-term measurements in UK remote/rural locations, although intensive campaigns have been undertaken at locations such as Mace Head2 (Ireland), and Weybourne3 (Norfolk). Long-term rural hydrocarbon measurements would have a variety of uses, for example providing information on the air quality arriving at urban locations, assessing the impact of city plumes and outflow on rural areas, and on the effect of European pollution events on the UK. The sole monitoring station in the AUN network classified as a rural monitoring station is sited at Harwell (Oxfordshire). Its location, within the highly urbanized southeast corner of the UK, makes it unrepresentative of rural concentrations within the UK as a whole.The University of Leeds field station at Haverah Park, located on open moorland, approximately 20 km to the north of Leeds, offers the opportunity to study a number of the above issues, dependent on the origin of air mass. Samples originating from the north and northwest will be relatively clean, having encountered few anthropogenic sources within the UK. Conversely, air masses originating from the south-southwest will bring relatively high concentrations of hydrocarbons from the Leeds–Bradford urban conurbation. During anti-cyclonic conditions, easterly airflows will bring in continental European air, without significant enhancement from major UK sources.
The relative concentrations of various hydrocarbons have been used to assess the chemical history of the incoming air mass. A variety of quantitative approaches can be used4,5 but they have a common qualitative approach, i.e. that as the air mass ages it is relatively enhanced in the more unreactive species. In most cases, OH is the most important species for hydrocarbon oxidation, but the influence of other initiating species such as Cl, Br, NO3 and O3 can be detected from such studies.6–8 During the winter, Haverah Park is mainly subject to northwesterly or southwesterly airflows, bringing in relatively clean and dirty air respectively and thus we would expect to see a significant difference in relative hydrocarbon concentrations if chemical processing has been taking place. The C6–C8 aromatic hydrocarbons considered in the study are ideal for this work as they are primarily attributed to a single source, automotive exhaust, and with a relatively constant initial distribution.9
This paper reports the concentrations of C6–C8 hydrocarbons, taken at approximately hourly intervals in a two-week period during January 1999 to assess the suitability of Haverah Park as a long-term monitoring station. Although the dataset is comparatively small, the weather patterns encountered during this period are representative of the entire winter and, therefore, we expect the observed concentrations to bare comparison with longer-term measurements from elsewhere in the UK. Variations in concentration levels can be rationalized from the trajectories of the parent air masses with both the short and long-term origin of the air mass contributing to the observed concentration levels. Ratios of hydrocarbon concentrations have been analysed in order to investigate the degree of chemical processing that has taken place during the air mass trajectory.
Site location and distribution of air trajectories
The University of Leeds field station (53° 58.2′ N, 1° 38.2′ W) is located on open moorland approximately 20 km north of Leeds (population ∼420,000) and 4 km to the southwest of Harrogate (population ∼70,000). The site is serviced by an access road (∼10 cars per day) and the nearest public road is 1 km to the south (peak flow ∼20 cars per h).Trajectory analyses were carried out for arrival every 6 h at Haverah Park between November 1998 and March 1999 using the Transport and Dispersion HYSPLIT (Hybrid Single-Particle Lagrangian Integrated Trajectory) model (http://www.arl.noaa.gov/ready/hysplit4.html). During the campaign the model was run every hour. Trajectories were assigned to one of eight sectors (each of 45°, centred on Haverah Park, sector 1 = 0°–45°, sector 2 = 45°–90°, etc.), primarily based on the trajectory over the past 24 h.
The trajectory data for the winter as a whole, and the intensive sampling campaign (5th–19th January), are presented in Table 1 and summarised in Fig. 1. There are two main types of trajectories arriving at Haverah Park: relatively polluted (sectors 4–6) and relatively clean (sectors 7–8). The local conurbations of Bradford and Leeds and also more distant sources in West Yorkshire and Lancashire will influence trajectories arriving from sectors 4–6. Trajectories from sectors 7 and 8 have no local major sources and few distant sources. Whilst there are slight discrepancies between the distributions with these two major groupings, the overall division between sectors 4–6 (‘dirty’) and sectors 7–8 (‘clean’) are very similar in the winter as a whole and during the measurement campaign.
 | ||
| Fig. 1 Windrose diagram showing the relative abundance of trajectories during the winter of 1998–9 (solid line) and during the intensive sampling campaign (dashed line). | ||
| Sector | Percentage of trajectories during winter 1998–9 | Percentage of trajectories during campaign | Comments |
|---|---|---|---|
| 1 | 3 | 2 | Potentially influenced by local emissions from Harrogate |
| 2 | 2 | 0 | |
| 3 | 2 | 0 | |
| 4 | 7 | 3 | Potenially mixed trajectories, Leeds only influences part of this sector |
| 5 | 27 | 41 | Virtually all trajectories originating from this sector are likely to have passed over Leeds or Bradford |
| 6 | 26 | 18 | Similar to sector 4, in that major local conurbations only influence part of this sector |
| 7 | 18 | 12 | No major local sources of pollution. Few major distant sources |
| 8 | 15 | 24 | |
| ‘Dirty’ sectors 4–6 | 60 | 62 | |
| ‘Clean’ sectors 7–8 | 33 | 36 |
Experimental
Air samples were collected at a height of 12 m over a 10 min period at a flow rate of 80 ml min−1 onto a Tenax TA trap (∼0.022 g) held at 0 °C by a Peltier cooler. Excess water was removed by passing the sample through a Dreschel flask immersed in chilled (−10 °C) ethylene glycol coolant. Once collected the sample was rapidly heated to 200 °C at 16 °C s−1 and the sample was introduced onto the column without further focussing. The column (60 m BP5, 0.53 mm id and a film thickness of 3 µm) was housed in a Carlo Erba HRGC-3000 which was used to temperature-programme the analysis. Upon injection the oven was held at 30 °C for 4 min and then heated at 10 °C min−1 to 75 °C where it remained for a further minute. A second, slower phase of heating increased the temperature to 155 °C at 4 °C min−1. After remaining at 155 °C for a further minute, the column was rapidly ramped at 20 °C min−1 to 200 °C and maintained at that temperature for 10 min in order to remove the more slowly eluting heavier-weight compounds. Once the analysis was complete the oven returned to 30 °C and the injector to 0 °C in preparation for the next sample. The overall cycle time was approximately 75 min.Peaks were identified both by retention times of known standards in the field and by mass spectrometric analysis in the laboratory. Calibrations were performed at the beginning and end of the campaign using known concentrations of ppbv standards (National Physical Laboratory).
Results and discussion
Absolute concentrations
Fig. 2 shows a time series of benzene over the duration of the campaign along with the associated trajectory. As would be expected, periods of relatively high hydrocarbon concentrations correspond to air masses originating from sectors 4–6, low concentrations from sectors 7 and 8. Fig. 3 shows an alternative representation of the data emphasising the role of local sources (Bradford, Leeds and Harrogate) in determining high hydrocarbon concentrations. | ||
| Fig. 2 Plot of absolute benzene concentrations (ppbv) and sector origins of air masses during the sampling campaign. | ||
 | ||
| Fig. 3 Average relative benzene concentrations as a function of sector demonstrating the influence of the local conurbations on benzene concentrations at the receptor site. | ||
The data for all hydrocarbons are summarised in Table 2, which also shows data for the campaign period from Harwell and East Birmingham (data were not available for the Leeds hydrocarbon site for the campaign period) as examples of the AUN rural and typical urban measurements. Concentrations of benzene and toluene at Haverah Park are significantly (at 95% confidence limit) lower than for Harwell. For the other hydrocarbons measured the large variability compared to the absolute values prevents a meaningful comparison, but concentrations at Haverah Park are comparable or lower than for Harwell and, as would be expected, much lower than the typical urban values.
| Benzene | n-Heptane | Toluene | Ethylbenzene | m,p-Xylene | o-Xylene | Benzene/toluene | Benzene/m,p-xylene | |
|---|---|---|---|---|---|---|---|---|
| Average/pptv | 110 | 30 | 280 | 50 | 140 | 50 | 0.6 | 1.2 |
| Standard deviation/pptv | 130 | 35 | 450 | 60 | 220 | 70 | 0.3 | 0.6 |
| Minimum/pptv | 10 | 5 | 15 | 5 | 10 | 5 | 0.1 | 0.3 |
| Maximum/pptv | 1020 | 300 | 4220 | 490 | 1760 | 560 | 1.9 | 4.0 |
| Harwell average/pptv | 300 | 42 | 360 | 51 | 173 | 48 | 1.23 | |
| Birmingham average/pptv | 1040 | 1920 |
Potentially more interesting than the average values at Haverah Park and Harwell is a comparison of the distributions at the lower concentrations. Approximately 60 data points were recorded at Haverah Park where benzene levels dropped below 50 pptv, none were recorded at Harwell. The minimum benzene level detected at Haverah Park is more than a factor of 5 below that recorded at Harwell. These results would suggest that, as might be expected given their relative locations in the UK, Haverah Park is subject to periods of much cleaner air than Harwell.
Correlations between hydrocarbons
Fig. 4 shows examples of typical correlations of various hydrocarbons with benzene. All of the aromatics are correlated at the 95% confidence interval as illustrated in Table 3 suggesting a common source for these hydrocarbons. Lower correlations (but still significant at the 95% level) were observed between n-heptane and the other species (e.g.Fig. 4(c)). The presence of relatively elevated n-heptane concentrations during periods of low absolute concentrations suggests the possibility of a local source, probably biogenic in origin. The variance analysis presented below further supports this view. | ||
| Fig. 4 Selected correlation plots of hydrocarbon concentrations. | ||
Monod et al.9 have made a detailed comparison of monoaromatic compounds in urban air and Derwent et al.1 have looked at a variety of hydrocarbon ratios with respect to benzene. We might expect to see deviations from urban ratios if significant chemical processing had taken place before the air mass arrived at our rural receptor, however, for most species the reported variability in urban ratios is too great to make any significant comparisons between our results and the measurements of Monod et al. and Derwent et al.
Chemical processing
Significant chemical processing should result in preferential removal of more reactive species. Evidence for this can be seen on detailed examination of the correlation plots shown in Fig. 4(a–c). The gradient of the plots of [hydrocarbon] vs. [benzene], the latter having the lowest rate coefficient for reaction with OH, decrease with decreasing benzene concentration, demonstrating the relative enhancement of the more unreactive benzene.Air originating from the cleaner sectors (7 and 8) will be less influenced by local emissions and the air will have undergone greater chemical processing. Fig. 5 shows a plot of [benzene], the benzene to m,p-xylene ratio and sector origin, vs. time. Periods of low benzene generally correlate with high benzene:m,p xylene ratios (high degree of chemical processing) and usually originate form sectors 7 and 8. Air masses originating from sectors 4–6, which will be more highly influenced by local emissions, have lower ratios. The effect of increased chemical processing can be seen more clearly in Fig. 6 where we plot the average ratios vs. sector for benzene:toluene and benzene:m,p-xylene. The ratios increase as we move from local, polluted air masses (sector 5), through sector 6, which contains mixed trajectories, to highly aged air masses, originating from distant sources in Sectors 7 and 8. As would be expected the gradient of the plot increases with the reactivity of compound with respect to benzene. Qualitatively the data seem to behave much as expected. Is it possible to extract more quantitative information?
 | ||
| Fig. 5 Variation of benzene:m + p-xylene ratio (solid line) and benzene concentration (squares) as a function of air mass origin (diamonds). Air originating from sectors 7 and 8 is relatively aged and hence has undergone preferential xylene removal. | ||
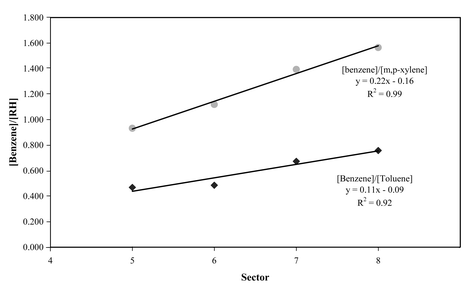 | ||
| Fig. 6 Averaged benzene:xylene (circles) and benzene:toluene (diamonds) ratios as a function of sector. | ||
Assuming that hydrocarbon removal can be represented by simple exponential decays, with removal of the hydrocarbon solely by reaction with OH and diffusion into a zero background, then the concentration of hydrocarbons, [RH], reaching Haverah Park from a particular sector are given by:
 | (1) |
 | (2) |
 | (3) |
 vs.ki,OH should be a straight line with gradient [OH]av
×
Δt. Such a plot, with rate coefficients taken from a recent evaluation,10 is shown in Fig. 7. The straight line obtained (R2
= 0.74) is consistent (at the 95% confidence level) with chemical processing by OH.
vs.ki,OH should be a straight line with gradient [OH]av
×
Δt. Such a plot, with rate coefficients taken from a recent evaluation,10 is shown in Fig. 7. The straight line obtained (R2
= 0.74) is consistent (at the 95% confidence level) with chemical processing by OH.
 | ||
| Fig. 7 Natural logarithm of ratios of hydrocarbon concentrations from dirty and clean sectors as a function of the rate coefficient for OH removal. | ||
The average hydroxyl radical concentration could be determined if Δt were known. Whilst it is possible to determine the average duration from source to receptor for sector 5 trajectories, sector 8 trajectories are much less certain. In many instances 5 day back trajectories (the limit for the HYSPLIT programme) for sector 8 do not pass over obvious sources, preventing a reliable estimation of trajectory times. In Table 4, average [OH] are shown calculated from a range of Δt. Few experimental determinations of winter OH concentrations exist with which to compare our data. Averaged global OH concentrations based on methyl chloroform measurements are of the order of 1 × 106 molecules per cm3.11 All our estimates are sensibly below this value, as would be predicted for winter when solar radiation levels are low. However, measurements made in Birmingham during the winter of 1999–2000 yielded surprisingly high concentrations of OH, greater than 1 × 106 molecules per cm3 indicating that in urban environments (and hence in urban plumes) additional mechanisms besides ozone photolysis exist for OH formation.12 Long-term measurements at Haverah Park of well defined urban plumes could help to address these issues.
| Δt | [OH]/molecule per cm3 |
|---|---|
| 2 days | 2.3 × 105 |
| 5 days | 9.3 × 104 |
| 10 days | 4.2 × 104 |
The value of calculations based on simple first-order physical and chemical removal of hydrocarbons has been questioned recently in the literature.13,14 Certainly, there are many ways in which such a model for the removal of hydrocarbons can be improved, however, given the uncertainties in trajectory analysis and the size of the data set, it is doubtful whether more sophisticated models would yield significantly more information.
Variance analysis
An alternative approach is to move away from a consideration of concentrations of hydrocarbons (relative or absolute) and focus on the variance of each hydrocarbon and its relationship with chemical lifetimes. Junge15 first proposed an inverse relationship between the residual standard deviation and chemical lifetime.| RSD = 0.14/τ | (4) |
| Sln[Xi] = Aτi−b | (5) |
| log (Sln[Xi]) = log A − b log τi | (6) |
Fig. 8(a) shows such a variance analysis for the complete data set with Fig. 8(b) and (c) showing the same plot for the well defined sectors 4,5 and 7,8 respectively. In each plot n-heptane shows significantly less variance than the other compounds, suggesting that a local source contributes to the observed concentrations, consistent with the positive intercepts on the n-heptane axis for the scatter plots described above. Calculations of the gradient and hence the b parameter exclude n-heptane.
 | ||
| Fig. 8 Variance plots vs. OH lifetimes for the complete campaign (a) and from well defined polluted (b) and clean (c) trajectories. | ||
Under conditions where the variance of the hydrocarbon concentrations is determined by variations in emissions (i.e. close to source), the dependence of variance with OH lifetime will be limited and b → 0. Conversely for well mixed and aged air, variations in source strengths are diminished and the variance is controlled by chemical removal and so b → 0. We have observed examples of the latter behaviour during measurements in the Arctic18 where b = 1.04, and of the former, where b = 0.06, for measurements in the urban background in Birmingham.19
The results from the current work at Haverah Park do not fit in with this relationship; b values from polluted (b = 0.08) and clean environments (b = 0.09) are both low, indicating that the variance is source dominated, and similar, despite the changes in concentration ratios indicating significant chemical processing in clean trajectories. The apparent anomaly between the two different analysis methods is difficult to reconcile. It may of course be due to the small datasets used and longer-term measurements may show no inconsistencies between the methodologies. However, as stated before, the air masses encountered were representative of the winter as a whole and so we wouldn't expect significant differences from a more extended campaign. Previous publications utilising variance analysis have used datasets of comparable size.16,20
Summary
Results from this work show Haverah Park is an interesting location for long-term measurements. Our initial campaign shows that a variety of air masses can be sampled at the site, yielding important information both on absolute concentrations, but also on the degree of chemical processing that occurs in both urban plumes and highly aged air masses.Hydrocarbon ratios show evidence of significant OH chemical processing during the winter, but the observed weak correlation of variance with OH chemical lifetime does not follow the expected pattern. Longer-term measurements will reveal whether this behaviour is reproducible and whether it has any seasonal dependence.
References
- R. G. Derwent, T. J. Davies, M. Delaney, G. J. Dollard, R. A. Field, P. Dumitrean, P. D. Nason, B. M. R. Jones and S. A. Pepler, Atmos. Environ., 2000, 34, 297 CrossRef CAS.
- A. C. Lewis, K. D. Bartle, D. E. Heard, J. B. McQuaid, M. J. Pilling and P. W. Seakins, J. Chem. Soc. Faraday Trans., 1997, 93, 2921 RSC.
- J. L. Grenfell, N. H. Savage, R. M. Harrison, S. A. Penkett, O. Forberich, F. J. Comes, K. C. Clemitshaw, R. A. Burgess, L. M. Cardenas, B. Davison and G. G. McFadyen, J. Atmos. Chem., 1999, 33, 183 CrossRef CAS.
- N. Balke, S. A. Penkett, K. C. Clemitshaw, P. Anwyl, P. Lightman and A. R. W. Marsh, J. Geophys. Res., 1993, 98, 2851 CAS.
- S. A. McKeen, M. Trainer, E.-Y. Hsie, R. K. Tallamraju and S. C. Liu, J. Geophys. Res., 1990, 95, 7493 CAS.
- O. W. Wingenter, D. R. Blake, N. J. Blake, B. C. Sive, F. S. Rowland, E. Atlas and F. Flocke, J. Geophys. Res., 1999, 104, 21819 CrossRef CAS.
- B. T. Jobson, H. Niki, Y. Yokouchi, J. W. Bottenheim, F. Hopper and R. Leaitch, J. Geophys. Res., 1994, 99, 25355 CrossRef.
- S. A. Penkett, N. J. Blake, P. Lightman, A. R. W. Marsh, P. Anwyl and G. Butcher, J. Geophys. Res., 1993, 98, 2865 CAS.
- A. Monod, B. C. Sive, P. Avino, T. Chen, D. R. Blake and F. S. Rowland, Atmos. Environ., 2001, 35, 135 CrossRef CAS.
- W. B. DeMore, S. P. Sander, D. M. Goldan, R. F. Hampson, M. J. Kurylo, C. J. Howard, A. R. Ravishankara, C. E. Kolb and M. J. Molina, JPL Publication 97-4, 1997 Search PubMed.
- R. G. Prinn, J. Huang, R. F. Weiss, D. M. Cunnold, P. J. Fraser, P. G. Simmonds, A. McCulloch, C. Harth, P. Salameh, S. O'Doherty, R. H. J. Wang, L. Porter and B. R. Miller, Science, 2001, 292, 1882 CrossRef CAS.
- J. D. Lee, PhD Thesis, University of Leeds, 2000.
- S. A. McKeen, S. C. Liu, E.-Y. Hsie, X. Lin, J. D. Bradshaw, S. Smyth, G. L. Gregory and D. R. Blake, J. Geophys. Res., 1996, 101, 2087 CrossRef CAS.
- D. H. Ehhalt, F. Rohrer, A. Whaner, M. J. Prather and D. R. Blake, J. Geophys. Res., 1998, 103, 18981 CrossRef CAS.
- C. E. Junge, Tellus, 1974, 26, 477 Search PubMed.
- B. T. Jobson, D. D. Parrish, P. Goldan, W. Kuster, F. C. Fehsenfield, D. R. Blake, N. J. Blake and H. Niki, J. Geophys. Res., 1998, 103, 13557 CrossRef CAS.
- B. T. Jobson, S. A. McKeen, D. D. Parrish, F. C. Fehsenfield, D. R. Blake, A. H. Goldstein, S. M. Schauffer and J. W. Elkins, J. Geophys. Res., 1999, 104, 16091 CrossRef CAS.
- J. R. Hopkins, A. C. Lewis, J. B. McQuaid and P. W. Seakins, Atmos. Environ., 2002, 36, 3217 CrossRef CAS.
- J. R. Hopkins, PhD Thesis, University of Leeds, 2001.
- J. Williams, H. Fischer, G. W. Harris, P. J. Crutzen, P. Hoor, A. Hansel, R. Holzinger, C. Warneke, W. Lindinger, B. Scheeren and J. Lelieveld, J. Geophys. Res., 2000, 105, 20473 CrossRef CAS.
Footnote |
| † Now at School of Environment, University of Leeds, Leeds, LS2 9JT. |
| This journal is © The Royal Society of Chemistry 2003 |
