Indenyl–ruthenium(ii) allenylidene complexes containing terpenic substituents as precursors of optically active terminal alkynes: scope and limitations†
Abstract
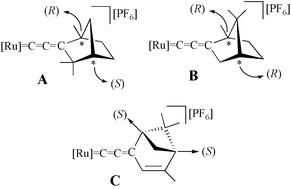
The optically active allenylidene complex [Ru{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C9H16)}(η5-C9H7)(PPh3)2][PF6]
(C(C9H16)
=
(1R,4S)-1,3,3-trimethyl-bicyclo[2.2.1]hept-2-ylidene)
1 regio- and stereoselectively reacts with unhindered anionic nucleophiles to yield the neutral σ-alkynyl derivatives [Ru{C
C(C9H16)}(η5-C9H7)(PPh3)2][PF6]
(C(C9H16)
=
(1R,4S)-1,3,3-trimethyl-bicyclo[2.2.1]hept-2-ylidene)
1 regio- and stereoselectively reacts with unhindered anionic nucleophiles to yield the neutral σ-alkynyl derivatives [Ru{C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(C9H16)R}(η5-C9H7)(PPh3)2]
(R = H 2a, C
CC(C9H16)R}(η5-C9H7)(PPh3)2]
(R = H 2a, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N 2b, Me 2c, C
N 2b, Me 2c, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh 2d). Protonation of 2a–d with HBF4·Et2O affords the cationic vinylidene complexes [Ru{
CPh 2d). Protonation of 2a–d with HBF4·Et2O affords the cationic vinylidene complexes [Ru{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)C(C9H16)R}(η5-C9H7)(PPh3)2][BF4]
3a–d, which can be easily demetalated, by treatment with
C(H)C(C9H16)R}(η5-C9H7)(PPh3)2][BF4]
3a–d, which can be easily demetalated, by treatment with ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C(C9H16)R 4a–d. The novel optically active indenyl–ruthenium(II) allenylidene complexes [Ru{
C(C9H16)R 4a–d. The novel optically active indenyl–ruthenium(II) allenylidene complexes [Ru{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C9H16)}(η5-C9H7)(PPh3)2][PF6]
(C(C9H16)
=
(1R,4R)-1,7,7-trimethyl-bicyclo[2.2.1]hept-2-ylidene)
9 and [Ru{
C(C9H16)}(η5-C9H7)(PPh3)2][PF6]
(C(C9H16)
=
(1R,4R)-1,7,7-trimethyl-bicyclo[2.2.1]hept-2-ylidene)
9 and [Ru{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C9H14)}(η5-C9H7)(PPh3)2][PF6]
(C(C9H14)
=
(1S,5S)-4,6,6-trimethyl-bicyclo[3.1.1]hept-3-en-2-ylidene)
10 have been prepared by
C(C9H14)}(η5-C9H7)(PPh3)2][PF6]
(C(C9H14)
=
(1S,5S)-4,6,6-trimethyl-bicyclo[3.1.1]hept-3-en-2-ylidene)
10 have been prepared by ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(C9H15)}(η5-C9H7)(PPh3)2]
11
(C(C9H15)
=
(1R,4R)-1,7,7-trimethyl-bicyclo[2.2.1]hept-2-en-2-yl).
CC(C9H15)}(η5-C9H7)(PPh3)2]
11
(C(C9H15)
=
(1R,4R)-1,7,7-trimethyl-bicyclo[2.2.1]hept-2-en-2-yl).

 Please wait while we load your content...
Please wait while we load your content...