Carbon–sulfur bond cleavage and reduction of thiols and dithioethers under oxidative conditions
Abstract
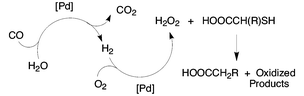
The reaction with hydrogen peroxide serves to cleave the carbon–sulfur bond and generate formally reduced products from certain thiols and dithioethers. The peroxide can be generated in situ from dioxygen using a supported palladium catalyst in the presence of a coreductant, either carbon monoxide or dihydrogen. The use of in situ generated oxidant provides a significant selectivity advantage compared to using a hydrogen peroxide solution. The reaction to form the reduced products is unique to compounds with a carboxylic group α to the carbon–sulfur bond.

 Please wait while we load your content...
Please wait while we load your content...