Hydration effects on the structural properties and haem–haem interaction in haemoglobin
Andrés G.
Salvay
ab,
Marcio F.
Colombo
*b and
J.
Raúl Grigera
*a
aInstituto de Física de Líquidos y Sistemas Biológicos, Departamento de Ciencias Biológicas, Universidad Nacional de La Plata, c.c. 565, B1900BTE, La Plata, Argentina. E-mail: grigera@iflysib.unlp.edu.ar; andres@iflysib.unlp.edu.ar; Fax: 54 221 425 73 17; Tel: 54 221 425 73 17
bDepartamento de Física, Instituto de Biociências Letras e Ciências Exactas, Universidade Estadual Paulista, Julio Mesquita Filho, 15054.000, São José do Rio Preto, SP, Brasil. E-mail: marcio@df.ibilce.unesp.br; Fax: 55 017 221 22 47; Tel: 55 017 221 22 45
First published on 27th November 2002
Abstract
Direct and simultaneous measurements of hydration water content and protein conformation have been performed using quartz crystal microbalance and visible absorption spectroscopy. Equilibrium and kinetics of methaemoglobin/haemichrome transition induced by the alteration of the degree of hydration was investigated in thin films exposed to controlled humidity. The kinetics experiment show that the conversion of species achieve the equilibrium more rapidly that the amount of sorbed water by the protein. The transition shows a sigmoid behaviour and suggest cooperative phenomena manifested by haem–haem interaction. The water hydration network contributing to the haem–haem interaction advise that water acts as allosteric effectors for the conversion between species. Irreversible changes produced by complete drying are clearly shown.
Introduction
The structural and functional behaviour of biomolecules is crucially dependent on their interactions with the solvent water. Ligand binding in allosteric proteins, DNA and RNA, enzyme to substrate association and catalyses, oligomerization or denaturation, are always followed by structural reorganization and consequently by water uptake or release. Because of a fraction of the chemical work done along these reactions is used to redistribute water molecules, the net change in the free energy of the reaction depends on the solution water activity.1 Therefore, changes in water activity itself are expected to shift the equilibrium of many biochemical processes.1Although several studies of the hydration of proteins have been undertaken and reviewed,2–5 very little is known about the molecular events produced during dehydration. Also the irreversibility of denaturation by drying is unclear. In order to achieve an improved understanding of the nature of the biological role played by water, the knowledge of the relation between the water content and macromolecular conformation and flexibility is essential. The present work is aimed in this direction.
Haemoglobin (Hb) is the obvious choice for the studies involving water-mediated transformation to complex proteins.1 Much of the current understanding of the allosteric mechanism and structure/function relation has resulted from studies on tetrameric α2β2 Hb.6,7
Protein films hydrated in the presence of different relative humidity are an appropriate case for these studies because practically all water molecules are close to a biomacromolecule.2 However, excepting a few number of works reported in the literature,8,9 water sorption experiments were performed on exhaustively dried samples, and thus losing the reference of the native state and consequently the information of the reversibility of the dehydration process.
Dehydration of myoglobin (Mb) films starting with aqueous solutions—or suspensions—, or from the high humidity range, show that when the protein is dried below a critical water content irreversible changes are produced in the macromolecule.8 Previous studies on Hb have shown that the dried protein forms a derivative optically10–14 and magnetically15,16 distinct from that observed in solution. This state is known as haemichrome or haemochrome depending if the haem iron is in the ferric or ferrous state respectively. It was proposed that in this derivative the iron is coordinated to the proximal and distal histidines;12,17,18 thus, the term bis–his has also been used to designate this derivative. In the metHb state, the sixth coordination position of each oxidized iron is occupied by a water molecule, while in the bis–his state this position is occupied by the imidazole-Nε of the distal histidine. Experiments made on Hb films shows that dehydration of Hb stabilize the bis–his state, which is characterized by the structural rigidity due to the absence of hydration water.19 However, it is unclear if dehydration produces irreversible changes in the protein. Besides, the kinetics of the water sorption and the relation between water content and conformation have not been done.
In this work we investigated the kinetics and equilibrium of the conformational transitions induced by the changes in water content of Hb films. We utilized quartz crystal microbalance (QCM) combined with visible absorption spectroscopy for the direct and simultaneous measurements of water content and the associate conformation respectively. With this methodology we studied the kinetics and equilibrium behaviour of the metHb/haemichrome transition as a function of the water content. The methodology allows an appropriate study of the molecular events produced during the water uptake or release and shows that complete dehydration produces irreversible changes in the protein.
Material and methods
Hb A0 in the oxy form was purified from human blood as previously described.20 The purified Hb was further stripped of organic and inorganic ions by several passages through an Amberlite MB-1 Sigma column, concentrated to about 8 mM Hb A0 using Amicon concentrators and stored in liquid N2 until use. The metHb derivative was prepared by addition of a very small excess of potassium ferricyanide to an oxyHb solution at neutral pH. The ferrocyanide formed was removed by gel filtration.21 The metHb sample solution was then concentrated to about 7 mM metHb. All chemicals were from Sigma.The water content of Hb film equilibrated at each relative humidity was determined with a QCM.22 The resonance frequency f of a quartz crystal resonator plate, cut at 35° to the crystallographic axis (AT plates), excited to thickness shear vibration, is extremely sensitive to changes on the mass m of thin films deposited on the faces of the crystal. The change on the mass Δm of a thin film deposited on area A of the quartz crystal electrodes causes a change Δf in frequency using the Sauerbrey equation Δf![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −(f2/ρqNfA)Δm, where f is the resonance frequency reference of the clean crystal, ρq the density of the quartz, and Nf a frequency constant.22 This linear relation between Δf and Δm is valid for acoustically thin films.23 For thick films and for measurements carried out with QCM operating in solution, the viscoelastic properties of the deposited film may produce an additional change in Δf besides rigid mass accumulation.24 We suggest that the isotherms of Hb hydration determined in this work agree, within experimental error, with others previously determined by gravimetric measurements.25,26 This evidences that the Sauerbrey equation is a good approximation to account for the frequency response of the QCM to Hb water uptake. The terms enclosed by parenthesis in the equation are a constant that depends on the crystal and determines its sensibility for mass determination. For the AT cut quartz crystals used in this work, Cristales Argentinos S.A., f
−(f2/ρqNfA)Δm, where f is the resonance frequency reference of the clean crystal, ρq the density of the quartz, and Nf a frequency constant.22 This linear relation between Δf and Δm is valid for acoustically thin films.23 For thick films and for measurements carried out with QCM operating in solution, the viscoelastic properties of the deposited film may produce an additional change in Δf besides rigid mass accumulation.24 We suggest that the isotherms of Hb hydration determined in this work agree, within experimental error, with others previously determined by gravimetric measurements.25,26 This evidences that the Sauerbrey equation is a good approximation to account for the frequency response of the QCM to Hb water uptake. The terms enclosed by parenthesis in the equation are a constant that depends on the crystal and determines its sensibility for mass determination. For the AT cut quartz crystals used in this work, Cristales Argentinos S.A., f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6000 kHz, A
6000 kHz, A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 cm2, Nf
1.2 cm2, Nf![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167 cm kHz, and ρq
167 cm kHz, and ρq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.65 g cm−3, giving a sensitivity of approximately 20 ng Hz−1.
2.65 g cm−3, giving a sensitivity of approximately 20 ng Hz−1.
The QCM was mounted into the closed optical cell shown in Fig. 1, which allows one to measure direct and simultaneously the weight of the Hb film deposited on the quartz crystal and the haemichrome content, via the visible spectrum of the Hb film deposited in an optical glass slide. In order to follow the formation of the films, less than 10 μl of a ∼7 mM metHb solution sample was spread on a glass slide and on the quartz crystal, which are arranged into the closed cylindrical cell. The bottom of the cell is filled with a volume of saturated salt solutions, which produces an atmosphere with different constant relative humidity (rh).27,28 The fully dry atmosphere was obtained with volume of P2O5 powder. The cell is positioned in a Cary 3E UV-Vis spectrophotometer for optical readings, and the quartz crystal connected to a resonator and a frequency meter which displays frequency to 1 Hz. The films were equilibrated at each rh until the haemichrome content and the hydration are equilibrated with the vapour phase, as judged by optical and weight measurements. This process takes about two hours for each rh. The full dehydration of the films was made by introduction of a volume of P2O5 powder into the closed cell for at least 12 h, to obtain the mass and the spectra of the fully dried sample. Using the Sauerbrey equation and the measured frequency difference between the dry sample and reference QCM, the mass of the dried film for a typical experiment was determinated to be 153.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02 μg. The intrinsic hydration h, given in units of g water per g protein, was evaluated taking the difference between the mass of the hydrated film and that of the fully dried film, and can be determinated to ±0.001 g water per g protein. Using the molecular weights of H2O and Hb, the h values can be converted to units of moles H2O per mol of Hb. The quantity of each derivative present in the film was evaluated from the optical absorbance in the visible range, since the several Hb derivatives have characteristic absorption bands in the 450–700 nm region.29 Measurements were made at 22
0.02 μg. The intrinsic hydration h, given in units of g water per g protein, was evaluated taking the difference between the mass of the hydrated film and that of the fully dried film, and can be determinated to ±0.001 g water per g protein. Using the molecular weights of H2O and Hb, the h values can be converted to units of moles H2O per mol of Hb. The quantity of each derivative present in the film was evaluated from the optical absorbance in the visible range, since the several Hb derivatives have characteristic absorption bands in the 450–700 nm region.29 Measurements were made at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
°C.
 | ||
Fig. 1 Scheme of the closed optical cell containing quartz crystal microbalance (QCM). Thin films of Hb are made coating the metHb solution sample on a glass slide and on the quartz crystal. The crystal frequency and the optical spectrum are taken simultaneously in order to determine the water content and the associate conformation respectively. Measurements are made at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. °C. | ||
Results
Kinetics of the metHb/haemichrome transition
Fig. 2 shows the simultaneous recording of the changes in the resonance frequency (a) and the optical absorption spectra (b) during the hydration of the film. Films of Hb are made on the quartz crystal and on a glass slide from the solution sample and exposed to an atmosphere of about 0.4 p/p0 (40% rh) for at least 60 min. Next the films were arranged into the closed optical cell containing a volume of saturated solution of K2SO4 to produce an atmosphere of 0.98 p/p0, and the data collection was started. The decrease in the frequency reports the amount of water taken by the protein along the hydration process. The evolution of the visible spectra during the hydration shows a decrease in intensity of the band at 536 nm and a simultaneous increase at 500 and 630 nm. The initial spectrum in Fig. 2b shows an absorption band at 536 nm and a shoulder near 565 nm, characteristic of the haemichrome observed in haemeproteins.11 The final spectrum shows absorption bands at 500 and 630 nm, typical of the metHb derivative in solution.29 In this way, the spectra indicate the tendency of the haemichrome conversion to metHb induced by the water uptake. The equilibrium was completed at 90 min, as judged by optical and frequency measurements. Another characteristic of the spectra shown in Fig. 2b is the presence of four isosbestic points at 485, 519, 595, and 660 nm, suggesting that only two derivatives, haemichrome and metHb, are present in the film. Isosbestic points very close to these were also observed in the metHb to haemichrome transition induced by dehydration,19 high pressure,18 freezing-dried,30 and by the addition of sodium salicylate in solution.31 | ||
| Fig. 2 Time evolution during the hydration at 0.98 p/p0 of (a) the resonant frequency change Δf of the quartz crystal coated with the Hb film, and (b) the spectra of the Hb film. | ||
Fig. 3 displays representative plots of the kinetics of water binding and metHb conversion in the film. The right coordinate displays the protein water content h as a function of the time of hydration. h is computed from the difference between the mass of the fully dehydrated film in an atmosphere dried with P2O5 and the mass of the hydrated protein, which are obtained from the changes Δf in the resonant frequency of the QCM. The fraction of metHb (fmetHb) in the film—displayed in the left coordinate—is calculated from the ratio R of the absorbance at 630 nm to that at 595 nm. In the absence of metHb, R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4, as obtained for the spectrum of the film equilibrated in an atmosphere dried with P2O5 after the kinetics measurements. R
0.4, as obtained for the spectrum of the film equilibrated in an atmosphere dried with P2O5 after the kinetics measurements. R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 corresponds to 100% metHb, as determined for the spectrum of the metHb solution sample. Even after reaching the equilibrium at the high humidity of 98% rh, a mixture of haemichrome and metHb is observed. Complete conversion was achieved only when water was dropped onto the film.
1.3 corresponds to 100% metHb, as determined for the spectrum of the metHb solution sample. Even after reaching the equilibrium at the high humidity of 98% rh, a mixture of haemichrome and metHb is observed. Complete conversion was achieved only when water was dropped onto the film.
 | ||
| Fig. 3 Kinetics of water sorption (h) and metHb content (fmetHb). fmetHb (○) is shown in the left axis and h (●) in the right axis. The lines connecting the experimental points are the best fit with a biexponential function to the h values (●) and with a single exponential to the fmetHb values (○). Fitting parameters are given in Table 1. | ||
| Process | τ y1/min | τ y2/min | τ y1/τy2 |
|---|---|---|---|
| Conversion to metHb (fmetHb) | 6.81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.17 0.17 |
0 | — |
| Water sorption (h) | 27.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.81 0.81 |
2.44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 0.12 |
11.37 |
The kinetics displayed in Fig. 3 shows that the conversion of haemichrome to metHb achieve the equilibrium more rapidly than the water uptake by the protein moiety. The data show that water sorption is biexponential time-dependent, while for the conversion of species only a single exponential is sufficient for fitting the experimental points. Table 1 shows the time constants of each process.
To better observe the relation between conformation and hydration, the data of Fig. 3 were plotted as the fraction of metHb against the water content (Fig. 4). At low water content the conversion of species is weakly responsive to increases in hydration. As water content is raised, the conversion of species steeply rises in response to small increases in water hydration; suggesting a cooperative behaviour.32 Preliminary results in films of monomeric Mb indicate a monoexponential time dependence for the conversion to haemichrome33 as well for the water sorption,34 indicating a non-sigmoid behaviour in the relation between conformation and water content.
 | ||
| Fig. 4 The change in the fraction of metHb (fmetHb) due to the change in water content (h). | ||
Sorption–desorption scanning experiments
We have also equilibrated samples in environments of different relative humidity. Fig. 5 shows the results obtained in these scanning experiments, where water sorption isotherms (Fig. 5a) are measured simultaneously with the fraction of metHb (fmetHb) in the film (Fig. 5b). The study of the water sorption–desorption cycles starts from the high humidity range. Initially, the films were made on the quartz crystal and on the glass slide by partially evaporation of a solution of the sample at 0.98 p/p0. After reaching the equilibrium the relative water vapour pressure was successively decreased to p/p0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08 and reversed to it original value of 0.98. When the water content is changed between h
0.08 and reversed to it original value of 0.98. When the water content is changed between h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.421 and hr
0.421 and hr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.063 g H2O per g Hb and backward the scanning cycle exhibits no hysteresis, and so the reversibility for the h and fmetHb values. However, below the critical hydration hr, hysteresis is observed both in the water sorption isotherm and in the curve showing the fraction metHb. The complete loop sorption–desorption for the Hb after complete drying shown in Fig. 5a is in good agreement with the water sorption isotherms reported in the literature.25,26 However, these water sorption isotherms previously reported were performed on exhaustively dried samples and thus losing the reference native state.
0.063 g H2O per g Hb and backward the scanning cycle exhibits no hysteresis, and so the reversibility for the h and fmetHb values. However, below the critical hydration hr, hysteresis is observed both in the water sorption isotherm and in the curve showing the fraction metHb. The complete loop sorption–desorption for the Hb after complete drying shown in Fig. 5a is in good agreement with the water sorption isotherms reported in the literature.25,26 However, these water sorption isotherms previously reported were performed on exhaustively dried samples and thus losing the reference native state.
 | ||
Fig. 5 Water sorption isotherms (a) measured simultaneously with the fraction of metHb (fmetHb) in the film (b). The experiment is started from the high humidity range. Dehydration below the critical value hr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.063 g H2O per g Hb (226 moles H2O per mol Hb) corresponding to pr 0.063 g H2O per g Hb (226 moles H2O per mol Hb) corresponding to pr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08 shows irreversibility as can be seen from the initial desorption of the native state from high hydration (■) and the complete loop of the sorption after dried (●) and desorption after dried and rehydration (□). The lines connecting the experimental points in (a) and (b) are the best fit with the Guggenheim isotherm formula and with the logistic Hill equation respectively. Fitting parameters are given in Tables 2 and 3 respectively. 0.08 shows irreversibility as can be seen from the initial desorption of the native state from high hydration (■) and the complete loop of the sorption after dried (●) and desorption after dried and rehydration (□). The lines connecting the experimental points in (a) and (b) are the best fit with the Guggenheim isotherm formula and with the logistic Hill equation respectively. Fitting parameters are given in Tables 2 and 3 respectively. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nc(p/p*)/{[1
Nc(p/p*)/{[1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (c
(c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)(p/p*)](1
1)(p/p*)](1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (p/p*)}
(p/p*)}
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (A1
(A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A2)/[1
A2)/[1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (p/kw)nw]
(p/kw)nw]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A2
A2
| Process | n w | k w | A 1 | A 2 |
|---|---|---|---|---|
| Initial desorption from high hydration | 13.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
0.88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02 0.02 |
0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
0.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 0.12 |
| Sorption after dried | 15.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7 2.7 |
0.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02 0.02 |
0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
0.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.07 0.07 |
| Desorption after dried and rehydration | 12.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
0.84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
0.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
Our results show that desorption isotherms starting from aqueous solutions or from the high humidity range represent true equilibrium conditions in the water content range h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hr. It is clear from Fig. 5 that once the protein is completely dried it behaves as a different material from the point of view of the sorption isotherms and of the conversion of species. Thus, hysteresis effects are consequence of complete water removal, which produces irreversible changes in the protein. For the initial relative water vapour pressure p/p0
hr. It is clear from Fig. 5 that once the protein is completely dried it behaves as a different material from the point of view of the sorption isotherms and of the conversion of species. Thus, hysteresis effects are consequence of complete water removal, which produces irreversible changes in the protein. For the initial relative water vapour pressure p/p0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.98 the water content is h
0.98 the water content is h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.421 g H2O per g Hb (1510 mol H2O per mol Hb) and the corresponding fraction of metHb fmetHb
0.421 g H2O per g Hb (1510 mol H2O per mol Hb) and the corresponding fraction of metHb fmetHb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.80. After complete dehydration and rehydration at the same 98% rh the values changes to h
0.80. After complete dehydration and rehydration at the same 98% rh the values changes to h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.395 g H2O per g Hb (1420 mol H2O per mol Hb) and fmetHb
0.395 g H2O per g Hb (1420 mol H2O per mol Hb) and fmetHb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.58. These results show that complete dehydration produces the formation of approximately 25% irreversible haemichrome. Also the complete conversion is not achieved when water was dropped onto the film.
0.58. These results show that complete dehydration produces the formation of approximately 25% irreversible haemichrome. Also the complete conversion is not achieved when water was dropped onto the film.
The three water sorption isotherms (Fig. 5a) were analyzed using the Guggenheim treatment of adsorption processes35 extended to biopolymers.36 In the Guggenheim isotherm, the total water uptake is given by h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ncp/p*/{[1
Ncp/p*/{[1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (c
(c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)p/p*](1
1)p/p*](1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p/p*)}, where N is the number of primary binding sites, p the relative water vapour pressure, c is a parameter related to the difference between the chemical potentials of the water in the primary binding sites and the bulk, and p* is a measure for the thermodynamic properties of the average multilayer water molecule.36,37 The parameter values fitted for the three isotherms are shown in Table 2, where we can see that the number N of primary binding sites decreases after the initial drying process, indicating irreversible changes after complete dehydration.
p/p*)}, where N is the number of primary binding sites, p the relative water vapour pressure, c is a parameter related to the difference between the chemical potentials of the water in the primary binding sites and the bulk, and p* is a measure for the thermodynamic properties of the average multilayer water molecule.36,37 The parameter values fitted for the three isotherms are shown in Table 2, where we can see that the number N of primary binding sites decreases after the initial drying process, indicating irreversible changes after complete dehydration.
The experimental data of the fraction of metHb in the film for each sorption–desorption cycle (Fig. 5b) were analyzed considering the conversion between two states induced by the uptake of nw water molecules and represented by bis–his-Hb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nw H2O
nw H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ⇆
⇆![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metHb. These data were fitted with the logistic Hill equation using fmetHb
metHb. These data were fitted with the logistic Hill equation using fmetHb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (A1
(A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A2)/[1
A2)/[1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (p/kw)nw]
(p/kw)nw]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A2, where A1 and A2 are constants, kw is the water association constant, and nw is the Hill constant representing the cooperativity of the transition which is less than the real number of water molecules involved in the transition.38 These parameters values are shown in Table 3.
A2, where A1 and A2 are constants, kw is the water association constant, and nw is the Hill constant representing the cooperativity of the transition which is less than the real number of water molecules involved in the transition.38 These parameters values are shown in Table 3.
Fig. 6 shows the fraction of metHb as a function of the water content. We can see that below a hydration ht of about 0.133 g H2O per g Hb (478 mol H2O per mol Hb) only haemichrome is present in the film. Above this value, where the percentage π of occupied primary binding sites approaches the values corresponding to saturation (see Table 4), haemichrome can be converted to metHb.
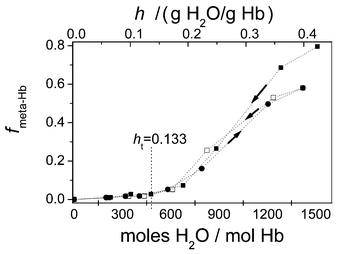 | ||
| Fig. 6 The change in the fraction of metHb (fmetHb) due to the change in water content (h) for the native state from high hydration (■) and the complete loop of the sorption after dried (●) and desorption after dried and rehydration (□). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100(h/N)(1
100(h/N)(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p/p*)37
p/p*)37
Discussion
The results described in this work suggest that metHb dehydration above the critical water content hr induces a reversible conformational change at the distal side of the haem group. This facilitates binding of the distal histidine to the haem iron in order to form the haemichrome derivative. The emerging picture is that dehydration induces a structural state in which the imidazole-Nε of the distal histidine is sterically accessible for binding to the haem iron ion, competing with the water for binding to the sixth coordination position. Dehydration of metHb below the critical water content hr produces formation of about 25% irreversible haemichrome.The kinetics of the metHb/haemichrome transition represented in Fig. 3 demonstrated a biphasic behaviour for the water sorption process. Such a biphasic behaviour is not manifested in the conversion of species which achieve equilibrium more rapidly than the amount of sorbed water by the protein. From this kinetics experiment starting at a water content of about ht and from the Hill constant nw calculated by fitting the data of Fig. 5b as displayed in Table 3, it can be shown that the uptake of a small quantity of water is sufficient to break the bond between the distal histidine and the haem iron. When this rupture is produced, the protein conformation relaxes to the typical conformation of metHb. This structural relaxation is accompanied by the binding of an additional number of water molecules. Preliminary results in monomeric Mb indicate monophasic kinetics for the haemichrome to metMb transition33 as well as for the water sorption process.34 Studies with Mossbauer spectroscopy39 and spectroscopic behaviour as a function of temperature40 indicate a different flexibility in the α- and β-chains of Hb. Thus, it is sensible to propose that the biphasic behaviour of the water sorption kinetics in Hb originates from the difference in flexibility between the α- and β-chains, although the possibility of some other heterogeneity in the protein cannot be excluded.
The sigmoid behaviour of the curves shown in Fig. 5b can be interpreted as if the binding of the distal histidine at the haem in one chain produces an increment in the capability to bind the other distal histidines at the other haem groups. Thus, cooperative phenomena are manifested. Optical and electron spin resonance spectral changes on reassociation of free met α- and β-subunits to form Hb tetramers show evidence for the presence of a major proportion of haemichrome in the free β-subunits, but this largely disappears on association with the α-subunits.41 Apparently this is because incorporation to the tetramers reverses the partial unfolding of the β-chains that is necessary for haemichrome formation.41 The spectral changes observed on the re-association of the subunits have been used by the authors as a basis for haem–haem interaction, and supports the interpretation that the sigmoid behaviour observed in Figs. 4, 5b and 6 reflects cooperative phenomena manifested by haem–haem interactions.
Several explanations for sigmoid kinetics are based on the idea that certain enzyme or protein molecules are composed of a number of subunits, which interact with one another defining allosteric interactions.42,43 These explanations also make reference to the allosteric effectors, which are bound to the macromolecule in a place different to the active site, influencing the affinity of the active site for the ligand. The fact that the affinity between distal histidine and haem iron is influenced by protein hydration suggests that water molecules act as allosteric effectors for the metHb/haemichrome transition. Water acting as allosteric effectors has been observed in the oxygenation of Hb.1 The experimental fact that the oxygen binding is accomplished by the uptake of water molecules points unambiguously to the predominant role of water in oxygen binding to Hb,1,44,45 and supports the idea that the network of water molecules hydrating the protein contribute to the haem–haem interaction.
Irreversible changes after complete drying are shown in Figs. 5 and 6. From these figures and from Table 4 we can see that for water content superior to ht—when the percentage π of occupied primary binding sites approaches values corresponding to saturation—haemichrome can be converted to metHb. This water content is necessary to keep the population of primary binding sites—in equilibrium with the multilayers—high enough to maintain the protein structure, a phenomenon that is well known for several proteins.8,9,46 Thus, the structure of metHb is sensitive to perturbations in the multilayers. Water content inferior to ht display a low occupancy of primary binding sites and only the haemichrome state is present. Therefore, the totally occupation of primary sorption sites gives the necessary flexibility for the conversion to metHb. Sorption parameters displayed in Table 2 for the water sorption isotherms show a decrease of the number of primary binding sites N after complete dehydration, probably due to the direct interaction of hydrogen binding sites that are bridged by water molecules in the native state. These results indicate that when the primary sorption sites become vacant irreversible changes are manifested in the Hb, producing a fraction of a new haemichrome species that cannot be converted to metHb after complete hydration. A further characterisation of the structure of this new species is necessary, and may provide a better understanding of the chemical event triggered by extensive dehydration and the loss of conformation reversibility upon rehydration. This information must be of great biotechnological interest.
We conclude that water hydration is strongly involved in the stabilization of Hb structures acting as heterotropic allosteric effectors in the conversion of species and that dehydration produces irreversible changes in the native macromolecule. It appears that water plays a key role maintaining the native structure of Hb and the functional connection between the haem groups. Since the structures of most proteins are sensitive to water content, the present mechanism may be important with respect to other binding proteins as well.
Acknowledgements
This work was partially supported by CONICET and ANPCyT from Argentina and the CAPES, CNPq and FUNDUNESP from Brazil. A. G. S. is sponsored by UNLP Argentina and PROSUL/CNPq Brazil process no 69.0190/2002-6. J. R. G. is member of the “Carrera del Investigador” of CONICET. M. F. C. is Researcher Fellow of CNPq. We thank N. E. Sanches-Fornillo for careful reading of the manuscript.References
- M. F. Colombo, D. C. Rau and V. A. Parsegian, Science, 1992, 256, 655 CAS.
- R. B. Gregory, in Protein Solvent Interactions, ed. R. B. Gregory, Marcel Dekker, New York, 1995, pp. 191–264 Search PubMed.
- J. A. Rupley and G. Careri, Adv. Protein Chem., 1991, 41, 37 Search PubMed.
- J. A. Rupley, E. Gratton and G. Careri, Trends Biochem. Sci., 1983, 8, 18 CrossRef CAS.
- I. D. Kuntz and W. Kauzmann, Adv. Protein Chem., 1974, 28, 239 Search PubMed.
- M. F. Perutz, A. J. Wilkinson, M. Paoli and G. G. Dodson, Annu. Rev. Biophys. Biomol. Struct., 1998, 27, 1 CrossRef CAS.
- J. R. H. Tame, Trends Biochem. Sci., 1999, 24(10), 372 CrossRef CAS.
- J. R. Grigera and I. G. Mogilner, Water Bridges in Myoglobin, in Biophysics of Water, ed. F. Franks, Wiley & Sons, Chichister, 1982, pp. 39–41 Search PubMed.
- M. Lüscher-Mattli, Biopolymers, 1982, 21, 403.
- F. Haurowitz, J. Biol. Chem., 1951, 193, 443 CAS.
- D. Keilin and E. F. Hartree, Nature, 1952, 170, 161 CAS.
- S. Maricic, G. Pifat and V. Pravidic, Ber. Bunsen-Ges. Phys. Chem, 1964, 68, 787 Search PubMed.
- S. Bohm and L. V. Abaturov, FEBS Lett., 1977, 77(1), 21 CrossRef CAS.
- G. M. Alter, J. Biol. Chem., 1983, 258(24), 14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 CAS.
960 CAS. - W. S. Caughey, W. Y. Fujimoto, A. J. Bearden and T. H. Moss, Biochemistry, 1966, 5(4), 1255 CrossRef CAS.
- G. C. Papaefthymiou, B. H. Huynh, C. S. Yen., J. L. Groves and C. S. Wu, J. Chem. Phys., 1975, 62(8), 2995 CrossRef CAS.
- M. F. Perutz, E. J. Heidner, J. E. Ladner, J. G. Beetlestone, C. Ho and E. F. Slade, Biochemistry, 1974, 13(10), 2187 CrossRef CAS.
- G. B. Ogunmola, A. Zipp, F. Chen and W. Kauzmann, Proc. Natl. Acad. Sci. USA, 1977, 74(1), 1 CAS.
- M. F. Colombo and R. Sanches, Biophys. Chem., 1990, 36, 33 Search PubMed.
- M. F. Colombo and G. O. Bonilla-Rodriguez, J. Biol. Chem., 1996, 271(9), 4895 CrossRef CAS.
- M. W. Neal and J. R. Florini, Anal. Biochem., 1973, 55(1), 328 CAS.
- M. G. Kennerley, Polymer, 1969, 10, 833 CrossRef CAS.
- M. V. Voinova, M. Jonson and B. Kasemo, Biosens. Bioelectron., 2002, 17, 835 CrossRef CAS.
- R. A. Etchenique and E. J. Calvo, Electrochem. Commun., 1999, 1, 167 CrossRef CAS.
- G. Brausse, A. Mayer, T. Nedetzka, P. Schlecht and H. Vogel, J. Phys. Chem., 1968, 72(9), 3098 CrossRef CAS.
- D. D. Eley and R. B. Leslie, Adv. Chem. Phys., 1968, 7, 238 Search PubMed.
- M. J. Schneider and A. S. Schneider, J. Membr. Biol., 1972, 9(2), 127 Search PubMed.
- Handbook of Chemistry and Physics, 62nd edn., 1981–1982, CRC Press, Inc Search PubMed.
- E. Antonini and M. Brunori, Hemoglobin and Myoglobin in their Reactions with Ligands, North-Holland Publ., Amsterdam, 1971 Search PubMed.
- T. Iizuka and M. Kotani, Biochem. Biophys. Acta, 1969, 194, 351 Search PubMed.
- E. A. Rachmilewitz, J. Peisach and W. E. Blumberg, J. Biol. Chem., 1971, 246(10), 3356 CAS.
- CH. R. Cantor and P. R. Schimmel, Biophysical Chemistry Part III: The Behaviour of Biological Macromolecules, W. H. Freeman & Co., New York, 1980, pp. 939–978 Search PubMed.
- M. F. Colombo, Dissertation, Instituto de Física e Química de São Carlos, Universidade de São Paulo, SP, Brazil, 1988.
- A. G. Salvay, Dissertation, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Argentina, 2001.
- E. A. Guggenheim, Applications of Statistical Mechanics, Clarendon Press, Oxford, 1966, pp. 186–206 Search PubMed.
- H. J. C. Berendsen, in Water: A Comprehensive Treatise, Part V, ed. F. Frank, Plenum Press, New York, 1975, pp. 293–349 Search PubMed.
- J. R. Grigera and H. J. C. Berendsen, Biopolymers, 1979, 18, 47 CrossRef CAS.
- A. V. Hill, J. Physiol., 1910, 40, 4.
- K. H. Mayo, D. Kucheida, F. Parak and C. J. Chien, Proc. Natl. Acad. Sci. USA, 1983, 80(17), 5294 CAS.
- A. Cupane, M. Leone, E. Vitrano and L. Cordone, Biopolymers, 1988, 27(12), 1977 CrossRef CAS.
- R. Banerjee, Y. Alpert, F. Leterrier and R. J. Williams, Biochemistry, 1969, 8(7), 2862 CrossRef CAS.
- J. Monod, J. Wyman and J. P. Changeux, J. Mol. Biol., 1965, 12, 88 CAS.
- D. E. Koshland, G. Nemethy and D. Filmer, Biochemistry, 1966, 5(1), 365 CrossRef CAS.
- A. G. Salvay, J. R. Grigera and M. F. Colombo, Biophys. J., 2001, 84, 1 Search PubMed.
- D. Bulone, I. D. Donato, M. B. Palma-Vittoreli and M. U. Palma, J. Chem. Phys., 1991, 94, 6816 CrossRef CAS.
- J. R. Grigera, J. Chem. Phys., 1979, 16, 2145.
| This journal is © the Owner Societies 2003 |
