Influence of phosphorus and vanadium additives in the development of surface acid catalytic properties of mesoporous alumina
Konstadina M.
Kolonia
,
Dimitris E.
Petrakis
and
Athanasios K.
Ladavos
*
Department of Chemistry, University of Ioannina, Ioannina, 45110, Greece
First published on 18th November 2002
Abstract
Alumino-phosphoro-vanadate catalysts of the general formula Al100PxVy, where x,y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 5, 10 and 20, have been prepared by co-precipitation of calculated amounts of Al(NO3)3·9H2O, H3PO4 and V2O5 dissolved in NH4OH, with ammonia solution at pH
0, 5, 10 and 20, have been prepared by co-precipitation of calculated amounts of Al(NO3)3·9H2O, H3PO4 and V2O5 dissolved in NH4OH, with ammonia solution at pH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.5. The resulting solids, after characterization by N2-porosimetry and XRD measurements, were checked for their surface acidity by ammonia–TPD and were also tested for their catalytic activity towards isopropanol decomposition. It was found that the addition of phosphorus increases the acid sites per square metre of the Al100PxVy solids and the same effect is also observed by the addition of vanadium. The catalytic activity of the solids with y
9.5. The resulting solids, after characterization by N2-porosimetry and XRD measurements, were checked for their surface acidity by ammonia–TPD and were also tested for their catalytic activity towards isopropanol decomposition. It was found that the addition of phosphorus increases the acid sites per square metre of the Al100PxVy solids and the same effect is also observed by the addition of vanadium. The catalytic activity of the solids with y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) <
<![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, expressed as molecules decomposed per surface acid site per second, shows maxima for catalysts with higher cumulative addition of phosphorus and vanadium, at those P plus V concentrations that result in amorphous solids while the onset of crystallization results in reducing the activity. The opposite behaviour is observed for catalysts with 20% V where the crystal phase of V2O5 is apparent in XRD right from the beginning but becomes gradually amorphous at high phosphorus addition at x
20, expressed as molecules decomposed per surface acid site per second, shows maxima for catalysts with higher cumulative addition of phosphorus and vanadium, at those P plus V concentrations that result in amorphous solids while the onset of crystallization results in reducing the activity. The opposite behaviour is observed for catalysts with 20% V where the crystal phase of V2O5 is apparent in XRD right from the beginning but becomes gradually amorphous at high phosphorus addition at x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.
20.
Introduction
The development of well characterized metallophosphate solids possessing tailor-made surface characteristics, like porosity and acidity, is of interest for technological as well as for biochemical reasons. The technology-orientated possibilities include the use of such solids as acid dehydration/hydration catalysts1 as selective adsorbents2 as well as in separation technologies.3On the other hand, the biochemically orientated interest stems from the participation of phosphorus in a very extended range of bio-solids like bones, teeth, scales and shells.4 For reasons which are related to minimization of diffusion limitations of reactants towards the surface of such solids and the maximization of the consequent surface reaction rates, such solids are often developed, both in technology and in nature, in porous forms which often possess a kind of hierarchy, either in their solid structure or in their porosity.5
Another property of such solids which affects their performance in technical applications like catalysis, as well as their behavior in biological processes like biomineralization, is their surface acidity.6,7 Therefore, the existence of controlled surface acidity in confined spaces of such porous metallophosphate solids offers the possibility to monitor such effects. We should mention that in the recent years various research groups have developed mesoporous oxo-metallo-phosphates,8–11 but these studies usually referred to the preparation and characterization of these solids.
In the context of an extended study program which has started some years ago, we have prepared and characterized from a structural,12 textural,13 surface,12(a),13(a) fractal14 and catalytic12(a),15 point of view various aluminophosphate solids, containing also added cations like iron12,14,15 chromium,12(a),14,15 vanadium13(b),16 and the first row transition elements.13(a)
The present study is an extension of some previous works13(b),16,17 referring to such phosphoro-vanado-aluminate solids and deals with the effect of the incorporation of phosphorus and vanadium into γ-Al2O3, as well as on the surface acidity and the catalytic dehydration activity of the obtained solids, using the iso-propanol decomposition as a probe reaction. Furthermore, another point is the scrutinization of the effect of amorphous or crystalline phases, of similar composition, on the catalytic activity.
Experimental and results
Preparation and characterization of specimens
The samples examined have the abbreviated formula Al100PxVy where x,y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 5, 10, 20. The whole formula involves substantial amounts of oxygen to balance the cations, but a precise oxide-based formula is not fully established so the abbreviated version is the most relevant in use.13(b),16,17 The preparation procedure was as follows: calculated amounts of Al(NO3)3·9H2O (Merck p.a.) and H3PO4
(Ferak p.a.) were dissolved in 250 ml of distilled water and V2O5 dissolved in 10 ml NH4OH, was finally added. Then, an ammonia solution (Ferak p.a.) was added dropwise under stirring up to pH
0, 5, 10, 20. The whole formula involves substantial amounts of oxygen to balance the cations, but a precise oxide-based formula is not fully established so the abbreviated version is the most relevant in use.13(b),16,17 The preparation procedure was as follows: calculated amounts of Al(NO3)3·9H2O (Merck p.a.) and H3PO4
(Ferak p.a.) were dissolved in 250 ml of distilled water and V2O5 dissolved in 10 ml NH4OH, was finally added. Then, an ammonia solution (Ferak p.a.) was added dropwise under stirring up to pH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.5 for 30 min. The gel formed was dried at 110
9.5 for 30 min. The gel formed was dried at 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 24 h. Since previous thermogravimetric studies17 had shown that such gels lose mass at around 300–350
°C for 24 h. Since previous thermogravimetric studies17 had shown that such gels lose mass at around 300–350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C but stabilize their mass above this temperature, heating in this region was carried out very slowly (1
°C but stabilize their mass above this temperature, heating in this region was carried out very slowly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1) in a tubular furnace under atmospheric conditions. The final firing temperature was set to 600
°C min−1) in a tubular furnace under atmospheric conditions. The final firing temperature was set to 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for a 6 h period. The samples prepared and some of their properties are listed in Table 2. For convenience, the samples will be referred to as PxVy throughout the rest of the article.
°C for a 6 h period. The samples prepared and some of their properties are listed in Table 2. For convenience, the samples will be referred to as PxVy throughout the rest of the article.
| Sample | Surface area (BJH)/m2 g−1 | Pore volume (BJH)/cc g−1 | Mean pore diameter/nm | Peak of PSDa/nm | FWHM/nm | Acid sites/1017 m−2 | E app/kJ mol−1 | |
|---|---|---|---|---|---|---|---|---|
| Low temperature | High temperature | |||||||
| a PSD, pore size distribution. | ||||||||
| Al100P0V0 | 201.3 | 0.43 | 8.5 | 6.54 | 2.5 | 6.54 | 107.7 | — |
| Al100P5V0 | 245.3 | 0.52 | 8.4 | 6.34 | 2.4 | 9.53 | 120.0 | — |
| Al100P10V0 | 319.8 | 1.19 | 14.0 | 9.14 | 4.4 | 10.35 | 116.4 | — |
| Al100P20V0 | 239.5 | 0.93 | 15.5 | 10.74 | 6.3 | 11.68 | 128.5 | — |
| Al100P0V5 | 270.5 | 0.45 | 6.6 | 5.44 | 2.1 | 10.98 | 65.3 | 135.8 |
| Al100P5V5 | 352.6 | 0.70 | 8.0 | 6.24 | 2.9 | 12.06 | 65.2 | 122.7 |
| Al100P10V5 | 386.1 | 1.15 | 11.9 | 9.24 | 4.6 | 21.37 | 93.8 | — |
| Al100P20V5 | 336.0 | 0.48 | 5.7 | 10.34 | 5.9 | 14.10 | 95.8 | — |
| Al100P0V10 | 301.9 | 0.50 | 6.6 | 5.24 | 2.1 | 12.19 | 23.6 | 141.6 |
| Al100P5V10 | 359.0 | 0.83 | 9.3 | 6.94 | 3.4 | 6.92 | 41.1 | 107.9 |
| Al100P10V10 | 321.5 | 1.11 | 13.8 | 10.24 | 5.4 | 17.76 | 47.2 | 101.3 |
| Al100P20V10 | 257.9 | 0.36 | 5.6 | 12.44 | 10.2 | 17.49 | 77.2 | — |
| Al100P0V20 | 176.7 | 0.46 | 10.3 | 8.14 | 5.2 | 24.05 | 83.4 | 27.6 |
| Al100P5V20 | 286.3 | 0.74 | 10.4 | 7.34 | 4.1 | 20.92 | 90.9 | 37.5 |
| Al100P10V20 | 305.2 | 1.02 | 13.4 | 9.24 | 6.0 | 18.99 | 60.5 | 86.7 |
| Al100P20V20 | 199.9 | 0.24 | 4.9 | 12.64 | 10.3 | 25.08 | 62.9 | 110.4 |
The characterization of these samples by several techniques has already been reported13(b),16,17 elsewhere and is therefore only briefly mentioned here. X-ray diffraction (XRD) has shown that the parent solid (P0V0) has the typical crystal structure of γ-Al2O3 (Table 1 and ref. 13(b)). An addition of phosphorus into the synthesis mixture results in the gradual formation of increased amounts of amorphous material. According to the XRD data the formation of crystalline AlPO4 takes place in P20Vy samples. Gradual incorporation of vanadium into the synthesis mixture decreases the crystallinity of the γ-Al2O3 phase, but this influence is much more weaker compared to that of phosphorus. For solids with 20% vanadium content, the V2O5 crystal phase is apparent.
| Sample | Detected crystal phases from XRD |
|---|---|
| Al100P0V0 | γ-Al2O3 |
| Al100P5V0 | Amorphous |
| Al100P10V0 | Amorphous |
| Al100P20V0 | AlPO4 |
| Al100P0V5 | Amorphous |
| Al100P5V5 | Amorphous |
| Al100P10V5 | Amorphous |
| Al100P20V5 | AlPO4 |
| Al100P0V10 | Amorphous |
| Al100P5V10 | Amorphous |
| Al100P10V10 | Amorphous |
| Al100P20V10 | AlPO4 |
| Al100P0V20 | γ-Al2O3, V2O5 |
| Al100P5V20 | V2O5 |
| Al100P10V20 | V2O5 |
| Al100P20V20 | AlPO4, V2O5 |
The pore size distribution measurements were carried using a Fisons Sorptomatic 1900 instrument. The characterization techniques included the determination of nitrogen adsorption–desorption isotherms from which the pore size distributions were also found. Each sample was degassed before measurement for 12 h at T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and P
°C and P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−3 Torr. The surface areas and porosity measurements (BJH method)18
(Fig. 1 and Table 2) showed that the addition of P at a constant concentration of V, and/or the addition of V at a constant concentration of P, increases the specific surface area and the pore volume. The maximum surface area and pore volume is obtained for a 15% cumulative addition of the two elements.
10−3 Torr. The surface areas and porosity measurements (BJH method)18
(Fig. 1 and Table 2) showed that the addition of P at a constant concentration of V, and/or the addition of V at a constant concentration of P, increases the specific surface area and the pore volume. The maximum surface area and pore volume is obtained for a 15% cumulative addition of the two elements.
 | ||
| Fig. 1 N2 adsorption–desorption isotherms at 77 K (right side) and the corresponding pore size distributions (left side). D is the pore diameter. | ||
Surface acidity measurements
The temperature-programmed desorption (TPD) of ammonia was used to evaluate the total (Lewis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Brönsted) surface acidity of the materials.10 Briefly, the procedure was as follows. The sample weighing about 0.5 g was put on the perforated bed of a silica tube with a diameter of 1 cm. The tube was connected to a gas chromatograph (Shimadzu GC-8A) with a thermal conductivity (TC) detector. Each sample was initially heated at 500
Brönsted) surface acidity of the materials.10 Briefly, the procedure was as follows. The sample weighing about 0.5 g was put on the perforated bed of a silica tube with a diameter of 1 cm. The tube was connected to a gas chromatograph (Shimadzu GC-8A) with a thermal conductivity (TC) detector. Each sample was initially heated at 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C in a flow of helium for 2 h, whereupon the temperature was reduced to the working level of 100
°C in a flow of helium for 2 h, whereupon the temperature was reduced to the working level of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Then the sample was saturated with dried ammonia by replacing for 30 min the flow of helium through the sample by a flow of dried ammonia. The weakly adsorbed ammonia was flushed out with helium flow (20 ml min−1) for 2 h at 110
°C. Then the sample was saturated with dried ammonia by replacing for 30 min the flow of helium through the sample by a flow of dried ammonia. The weakly adsorbed ammonia was flushed out with helium flow (20 ml min−1) for 2 h at 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. After stripping of the weakly adsorbed ammonia the sample was cooled to 100
°C. After stripping of the weakly adsorbed ammonia the sample was cooled to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and heating begun at a rate of 10
°C and heating begun at a rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 till 500
°C min−1 till 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C where it remained for 2 h. The amount of ammonia eluted was trapped in a bubble bottle, which contained an excess of 0.01 N hydrochloric acid solution. The trapped ammonia was determined by volumetric titration of the excess HCl using 0.05 N NaOH. The results are summarised in Table 2, as surface acid sites per square metre of the sample.
°C where it remained for 2 h. The amount of ammonia eluted was trapped in a bubble bottle, which contained an excess of 0.01 N hydrochloric acid solution. The trapped ammonia was determined by volumetric titration of the excess HCl using 0.05 N NaOH. The results are summarised in Table 2, as surface acid sites per square metre of the sample.
Catalytic decomposition of isopropanol
The catalytic decomposition of isopropanol was performed in a bench-scale flow reactor, similar to that described previously.3(b),13(a) The reactor consisted of a silica tube with a diameter of 1 cm with a sealed-in quartz bed onto which 0.25 g of catalyst was placed. The system was heated in a tubular furnace with a temperature accuracy within ±0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Analyses of the reactants and products were carried out by sampling 1 cm3 of gases in a Shimadzu GC-15A gas chromatograph equipped with a thermal conductivity detector and connected to a Chromatopac C-R6A integrator. The column used for analysis was 2 m
°C. Analyses of the reactants and products were carried out by sampling 1 cm3 of gases in a Shimadzu GC-15A gas chromatograph equipped with a thermal conductivity detector and connected to a Chromatopac C-R6A integrator. The column used for analysis was 2 m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/8″ stainless steel filled with 10% Carbowax 20M on Chromosorb W-HP (80–100 mesh). Helium was used as carrier gas in the GC. Another line drove helium through a saturator bottle (40
1/8″ stainless steel filled with 10% Carbowax 20M on Chromosorb W-HP (80–100 mesh). Helium was used as carrier gas in the GC. Another line drove helium through a saturator bottle (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cm3 min−1) containing isopropanol. The partial pressure of isopropanol was 46 mbar. The experiments were carried out randomly in the temperature range 100–200
1 cm3 min−1) containing isopropanol. The partial pressure of isopropanol was 46 mbar. The experiments were carried out randomly in the temperature range 100–200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C at 5
°C at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C intervals. Before the catalytic experiments, all solids were heated at 200
°C intervals. Before the catalytic experiments, all solids were heated at 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 2 h in a helium flow. No deactivation was observed during the scale of our experiments. The products detected were propene and di-isopropyl ether. The reaction rate at each temperature was calculated from the degree of conversion of isopropanol, and the selectivity for each main product at various degrees of conversion of isopropanol was also found.
°C for 2 h in a helium flow. No deactivation was observed during the scale of our experiments. The products detected were propene and di-isopropyl ether. The reaction rate at each temperature was calculated from the degree of conversion of isopropanol, and the selectivity for each main product at various degrees of conversion of isopropanol was also found.
The corresponding integral reaction rates per square metre of the catalyst are shown in Fig. 2 as a function of temperature. In Fig. 3 the selectivity towards propene (Sp) and di-isopropyl ether (Se) is shown as a function of degree x of conversion.
 | ||
| Fig. 2 Temperature profiles of the catalytic activity (moles s−1 m−2) for the isopropanol decomposition. | ||
 | ||
| Fig. 3 Selectivity for the productions of propene (a) and of di-isopropyl-ether (b) as a function of conversion of isopropanol. | ||
Discussion
The addition of phosphorus increases the acid sites per square metre of the PxVy solids and the same effect is also observed with the addition of vanadium. The maximum number of acid sites occurs for the P20V20 sample. Similar results referring to continuous increase of number of acid sites with the addition of phosphorus have also been reported elsewhere.19,20 According to Morales et al.20 the adsorption of phosphorus, in the form of PO43−, on the surface of γ-Al2O3, leads to duplication of the number of acid sites per atom Al for every P atom added. As reported in ref. 16, the incorporation of vanadium is not as straightforward as that of phosphorus and XRD measurements have shown that it induces loss of crystallinity of the γ-Al2O3 structure. Therefore vanadium interacts with the framework. Furthermore, MAS NMR experiments showed that a small percentage of vanadium (especially for the samples with 20% V) is heterogeneously spread over the surface in the form of small clusters of V2O5-like species, which may originate from the nature of the vanadium source, i.e. V2O5, not having reacted with the alumina.16 Al MAS spectra indicated the appearance of amorphous AlPO4 for the lowest amount of phosphorus. Increasing the amount phosphorus causes the growth of this amorphous phase towards the inner part of the framework. The cumulative addition of phosphorus and vanadium led to the additional formation of crystalline AlPO4. The amorphous AlPO4 phase was composed of phosphoric species having at least three aluminium atoms in the second sphere of their coordination, near the surface of the pores. Both phosphorus and the rest of vanadium species are located near the surface of γ-Al2O3, probably forming Al–O–M bonds where M![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) P, V.16 Therefore the synergistic action of the two elements in the formation of acid sites is the reason for the increment of the acidity with the cumulative addition of phosphorus and vanadium.
P, V.16 Therefore the synergistic action of the two elements in the formation of acid sites is the reason for the increment of the acidity with the cumulative addition of phosphorus and vanadium.
In Fig. 2 it is shown that for the solids without vanadium, the addition of 5% P decreases the reaction rate. However, addition of phosphorus up to 20% improves the catalytic activity. For a small addition of vanadium (5% V) an increase in the catalytic activity is observed. Further addition of vanadium leads to a plateau in the reaction rate at a reaction temperature of 140–160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, but at higher reaction temperatures, which results in an increment of the catalytic activity. For the samples with 20% vanadium content, the addition of phosphorus decreases the reaction rate. Moreover, it is clear that solids with 10% and 20% vanadium show higher activity at low reaction temperatures, as compared to the other solids.
°C, but at higher reaction temperatures, which results in an increment of the catalytic activity. For the samples with 20% vanadium content, the addition of phosphorus decreases the reaction rate. Moreover, it is clear that solids with 10% and 20% vanadium show higher activity at low reaction temperatures, as compared to the other solids.
In Fig. 3 it is shown that the selectivity to propene is decreased for solids without vanadium and for degrees of conversion up to 15%. However, at higher degrees of conversion the selectivity to propene is increased. The addition of phosphorus leads to increment of selectivity to propene and reduction on the selectivity to di-isopropyl ether. For the solids which contain vanadium, a steep and continuous increment of the propene selectivity is observed. In general, propene is the main product of the dehydration reaction with a selectivity of at least fifty percent. For samples with vanadium and at low degrees of isopropanol conversion, a very low production of acetone is observed. According to Bond et al.21 the production of acetone via dehydrogenation is due to the presence of vanadyl groups (V![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) on the surface of the solids. The existence of such groups on our catalysts have been proved by solid state NMR measurements as presented above, and published in the past.16
O) on the surface of the solids. The existence of such groups on our catalysts have been proved by solid state NMR measurements as presented above, and published in the past.16
The rate of dehydration, which is an acid catalysed reaction, should increase parallel to the surface density of sites. However, this was not observed for all the examined solids. Therefore, it is plausible that parameters other than the number of acid sites could be critical in determining their apparent activity. Indeed, in Fig. 4a we observe that the activity of the solids (molecules decomposed per surface acid site per second) shows maxima for the catalysts with higher cumulative addition of phosphorus and vanadium, exactly at those P plus V concentrations that result in amorphous solids (compare with XRD data in Table 1). The sample Al100P0V0 is excluded. The opposite behaviour is observed for catalysts with 20% vanadium content. The differentiated behavior of the samples Al100PxV20 as far as Eapp, as well as the Selectivity concern, can be only due to the different crystal phases existing in the samples, which differentiate their surface acidity.
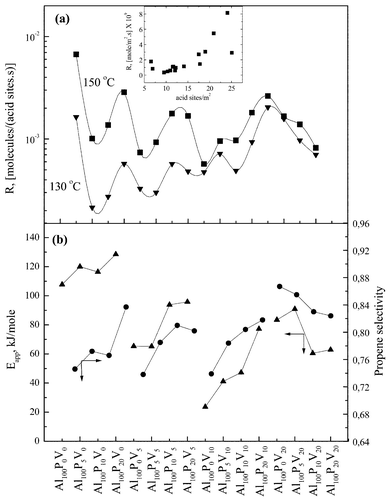 | ||
Fig. 4 Variation of (a) catalytic activity at 130 and 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C expressed as molecules decomposed per surface acid site per second; in an including graph the rate of dehydration as a function of the number of acid sites is shown. (b)
Eapp values for low reaction temperatures (▲) and selectivity to propene (●). °C expressed as molecules decomposed per surface acid site per second; in an including graph the rate of dehydration as a function of the number of acid sites is shown. (b)
Eapp values for low reaction temperatures (▲) and selectivity to propene (●). | ||
As far as the kinetics of isopropanol decomposition is concerned, we consider 1st order kinetics which is the simplest and most common treatment applied to this reaction.15,22–24
R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k θiso k θiso | (1) |
θiso![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (bisoPiso)/(1 (bisoPiso)/(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΣkiPi) ΣkiPi) | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≫
≫![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΣkiPi), eqn. (1) obtains the form
ΣkiPi), eqn. (1) obtains the form R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k bisoPiso k bisoPiso | (3) |
Then, expressing the partial pressure of isopropanol as a function of its initial pressure P0iso, of the degrees of conversion, the selectivities of every product and the total pressure in the reactor Pt, as well as using the design-equation of the plug flow reactor (F dx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R dS, where F is the feed (moles s−1) and S the surface area of the catalyst) as the one used in our case, we obtain after lengthy but rather trivial transformations eqn. (4)
(all the above transformations are reported in detail in ref. 24).
R dS, where F is the feed (moles s−1) and S the surface area of the catalyst) as the one used in our case, we obtain after lengthy but rather trivial transformations eqn. (4)
(all the above transformations are reported in detail in ref. 24).
 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727.5 mmHg, P0iso
727.5 mmHg, P0iso![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.16 mmHg, in our experiment we can easily rearrange eqn. (4) in the form
33.16 mmHg, in our experiment we can easily rearrange eqn. (4) in the form  | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A exp(−Eapp/RT), Etrue is the true activation energy of the reaction, biso
A exp(−Eapp/RT), Etrue is the true activation energy of the reaction, biso![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[(−ΔHiso)/RT], −ΔHiso is the enthalpy of adsorption of isopropanol, and Eapp is the apparent activation energy of the process related to Etrue and ΔH by the relationship Eapp
exp[(−ΔHiso)/RT], −ΔHiso is the enthalpy of adsorption of isopropanol, and Eapp is the apparent activation energy of the process related to Etrue and ΔH by the relationship Eapp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Etrue
Etrue![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔHiso.
ΔHiso.
Then, plots of the left hand part of eqn. (5)versus 1000/T can be used to obtain the apparent activation energy of the reaction Eapp. Such plots are shown in Fig. 5. The calculated numerical values of Eapp are listed in Table 2.
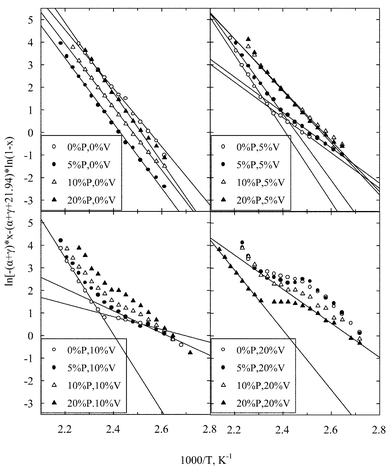 | ||
| Fig. 5 Arrhenius plots for the isopropanol decomposition drawn according to eqn. (5). | ||
From the Arrhenius lines drawn in Fig. 5 it is immediately apparent that the vanadium containing catalysts behave in a totally different way, as compared to the samples without vanadium. Those plots indicate two regions of activation energy: The first one appears at high temperatures and high conversions and the other at low reaction temperatures and low conversions of isorpopanol. We believe that the two regions of activation energy which appear at higher temperatures are due to the alternation of kinetics. The first order kinetic eqn. (3) does not hold for the whole range of conversion. There are two possibilities. The first one is that isopropanol itself is strongly adsorbed at low T and low conversions on Al100PxVy. If so, the kinetics should be zero order R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k and the apparent activation energy of the reaction Eapp should equal Etrue. The other possibility is that water, when produced in large quantities at higher conversion, acts inhibitively. Then, the apparent activation energy of the reaction should appear increased by an amount equal to that of the heat of water adsorption i.e.
k and the apparent activation energy of the reaction Eapp should equal Etrue. The other possibility is that water, when produced in large quantities at higher conversion, acts inhibitively. Then, the apparent activation energy of the reaction should appear increased by an amount equal to that of the heat of water adsorption i.e.
Eapp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Etrue Etrue![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔHC3H8O ΔHC3H8O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔHH2O ΔHH2O |
Eapp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Etrue Etrue![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔHC3H8O. ΔHC3H8O. |
In other words, the difference between the two sets of activation energies on Al100PxVy corresponds to the enthalpy of adsorption of water on the catalyst surface.
This peculiar behavior might also be connected with the heterogeneous spread of vanadium over the surface as small clusters of V2O5-like species, which affects the adsorption of reactant or/and products on the surface, and alters the reaction rate, the selectivity and the apparent activation energy of the reaction.
In Fig. 4b the values of Eapp for low reaction temperatures and the selectivity to propene are depicted for the four groups of solids. The estimated apparent activation energies run parallel to the selectivity to the main product, i.e. propene. As mentioned above, the calculated values of Eapp are related to the true activation energy Etrue and the enthalpy of adsorption of isopropanol ΔHiso. Catalysts with high surface acidity are expected to show higher catalytic activity, as well as higher negative values of ΔHiso and as a result higher Eapp values. For this reason, Eapp runs parallel to the selectivity of propene which is the main product of the reaction.
Plotting the values of the pre-exponential factor versus the corresponding activation energies, a perfectly straight line is obtained (Fig. 6) which corresponds to a single compensation effect. According to Patterson et al.,25 this happens because the variation of the strength of adsorption of the reactants and products affects the entropy of adsorption on the catalyst's surface. Arrhenius plots that obey the compensation effect must intersect at exactly the same point defining an “isokinetic temperature” and “isokinetic rate”. The isokinetic and compensation parameters are related to each other as follows:
Compensation effect : ln A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mEapp mEapp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C | (6) |
Isokinetic parameters : Tiso![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (mR)−1; ln(kiso) (mR)−1; ln(kiso)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C | (7) |
 | ||
| Fig. 6 Compensation effect for the isopropanol decomposition over the Al–P–V catalysts. | ||
From eqn. (7) and the estimated slope of the compensation effect line (Fig. 6) an “isokinetic temperature” of 424 K is calculated. It is remarkable that this value corresponds exactly to the reaction temperature where a changing of slope is observed at the Arrhenius plots in Fig. 5. Beyond this temperature, another reaction mechanism probably takes over, as discussed previously, with differentiated Eapp and lnA. Therefore some compensation perhaps holds there which cannot be determined from the present set of data.
Conclusions
The following conclusions can be drawn from this work.(i) The addition of phosphorus on γ-Al2O3 increases the acid sites per square metre of the Al100PxVy solids and the same effect is also observed with the addition of vanadium.
(ii) The main products of the isopropanol decomposition were propene and di-isopropyl ether, with selectivity to propene between 50 and 100%.
(iii) We did not observe any direct correlation between the reaction rate and the number of acid sites for all tested catalysts. The samples with medium and high vanadium content showed a rather peculiar behavior, which might be due to poor dispersion of vanadium on the surface, forming V2O5-clusters.
(iv) The catalytic activity of the solids expressed as molecules decomposed per surface acid site per second shows maxima for the catalysts with the higher cumulative addition of phosphorus and vanadium, exactly at those P plus V concentrations that result in amorphous solids. The opposite behaviour was observed for the catalysts with 20% vanadium content.
(v) From the Arrhenius plots it was apparent that vanadium containing catalysts showed two regions of activation energy. The reaction temperature, which separated those regions, corresponds exactly to the isokinetic temperature of the reaction, as it was calculated from the compensation effect.
Acknowledgements
The authors thank Professor P. J. Pomonis for the helpful comments and discussions.References
- (a) M. J. Campelo, M. F. Bautista, A. Garcia, D. Luna, M. J. Marinas and A. A. Romero, Appl. Catal., A, 1998, 166, 39–45 CrossRef; (b) Robert A. W. Johnstone, Jun-Yao Liu and David Whittaker, J. Mol. Catal. A: Chem., 2001, 174, 159–168 CrossRef; (c) M. C. Fougret and F. W. Hölderich, Appl. Catal., A, 2001, 207, 295–301 CrossRef.
- (a) K. G. Varshney, A. H. Pandith and U. Gupta, Langmuir, 1998, 14(26), 7353–7358 CrossRef CAS; (b) Y. Makita, H. Kanoh, T. Hirotsu and K. Ooi, Chem. Lett., 1998, 1, 77–78 CrossRef.
- (a) K. O. Miyai, Y. Makita and H. Kanoh, Sep. Sci. Technol., 1999, 34(6–7), 1133–1144 Search PubMed; (b) D. M. Kaschak, S. A. Johnson, C. C. Waraksa, J. Pogue and T. E. Mallouk, Coord. Chem. Rev., 1999, 186, 403–416 CrossRef; (c) S. Oliver, A. Kuperman and A. G. Ozin, Angew Chem., Int. Ed. Engl., 1998, 37, 46–62 CrossRef CAS.
- (a) S. Oliver, A. Kuperman, N. Coombs, A. Lough and A. G. Ozin, Nature, 1995, 378, 47–50 CrossRef CAS; (b) A. Stoch, W. Jastzebski, J. Stoch, J. Szaraniec, B. Trybalska and G. Kmita, J. Mol. Struct., 2000, 555, 375–382 CrossRef CAS; (c) M. Nagano, T. Nakamura, T. Kokubo, M. Tanahashi and M. Ogawa, Biomaterials, 1996, 17, 1771–1777 CrossRef CAS.
- (a) A. G. Ozin, Acc. Chem. Res., 1997, 30, 17–27 CrossRef CAS; (b) A. Choudhury, S. Natarajan and C. N. Rao, Inorg. Chem., 2000, 39, 4295–4304 CrossRef CAS.
- K. Tanabe, M. Misono, Y. Ono and Hattori, Studies in Surface Science and Catalysis, ed. B. Delmon and T. J. Yates, vol. 51, Amsterdam–Oxford–New York–Tokyo, 1990 Search PubMed.
- (a) L. M. Cornaglia, E. A. Lombardo, J. A. Andersen and J. J. Garcia Fierro, Appl. Catal., 1993, 100, 37–50 CrossRef CAS; (b) N. S. Pavlova, A. V. Sadykov, V. G. Zabolotnaya, I. D. Kochubey, I. R. Maximovskaya, I. V. Zaikovskii, V. V. Kriventsov, V. S. Tsybulya, B. E. Burgina, M. A. Volodin, V. M. Chaikina, N. N. Kuznetsova, V. V. Lunin, D. Agrawal and R. Roy, J. Mol. Catal. A: Chem., 2001, 174, 159–168 CrossRef; (c) A. H. G. Mekhemer, K. H. A. Nohman, E. N. Fouad and A. H. Khalaf, Colloids Surf., A, 2000, 161, 439–446 CrossRef CAS.
- U. Ciesla, S. Schacht, D. G. Stucky, K. K. Unger and F. Schuth, Angew. Chem., Int. Ed. Engl., 1996, 35(5), 541–543 CrossRef CAS.
- (a) S. M. Wong, M. D. Antonelli and Y. J. Ying, Nanostruct. Mater., 1997, 9, 165–168 CrossRef CAS; (b) E. Rodríguez-Castellón, J. Jiménez-Jiménez, A. Jiménez-López, P. Maireles-Torres, R. J. Ramos-Barrado, J. D. Jones and J. Rozière, Solid State Ionics, 1999, 125, 407–410 Search PubMed.
- L. M. Rodriguez, J. Alcaraz, M. Hernandez, M. Dufaux, Y. Ben Taarit and M. Vrinat, Appl. Catal., A, 1999, 189, 53 CrossRef CAS.
- K. J. Bartley, J. I. Ellison, A. Delimitis, J. C. Kiely, A.-Z. Isfahani, C. Rhodes and J. G. Hutchings, Phys. Chem. Chem. Phys., 2001, 3, 4606–4613 RSC.
- (a) D. E. Petrakis, P. J. Pomonis and A. T. Sdoukos, J. Chem. Soc., Faraday Trans. 1, 1989, 85(10), 3173–3186 RSC; (b) E. D. Petrakis, M. J. Hudson, J. P. Pomonis, A. T. Sdoukos and T. V. Bakas, Colloids Surf., A, 1994, 90, 191 CrossRef CAS; (c) D. E. Petrakis, M. J. Hudson, P. J. Pomonis, A. T. Sdoukos and T. V. Bakas, J. Mater. Chem., 1995, 5(11), 1975–1983 RSC.
- (a) D. E. Petrakis, P. J. Pomonis and A. T. Sdoukos, J. Chem. Soc., Faraday Trans., 1991, 87(6), 901–905 RSC; (b) K. M. Kolonia, D. E. Petrakis, T. N. Angelidis, P. N. Trikalitis and P. J. Pomonis, J. Mater. Chem., 1997, 7(9), 1925–1931 RSC.
- Petrakis E. Dimitris, Pashalidis Ioannis, Theocharis R. Charis, Hudson J. Michael and Pomonis J. Philip, J. Colloid Interface Sci., 1997, 185, 104–110 CrossRef CAS.
- D. E. Petrakis, P. J. Pomonis and A. T. Sdoukos, J. Chem. Soc., Faraday Trans., 1991, 87(9), 1439–1445 RSC.
- R. D. Gougeon, P. R. Bodart, R. K. Harris, D. M. Kolonia, D. E. Petrakis and P. J. Pomonis, Phys. Chem. Chem. Phys., 2000, 2, 5286–5292 RSC.
- K. M. Kolonia, D. E. Petrakis, T. C. Vaimakis, E. D. Economou and P. J. Pomonis, Thermochim. Acta, 1997, 293, 93–100 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373 CrossRef.
- D. Chadwick, D. W. Aitchison, R. Badilla-Ohtbaum and L. Josefsson, in Preparation of Catalysis III, Studies in Surface Science and Catalysis, vol. 16, Elsevier, Amsterdam, 1983, p. 323 Search PubMed.
- A. Morales, M. M. Ramirez de Angelo and F. Hernandez, Appl. Catal., 1988, 41, 261 CrossRef CAS.
- G. C. Bond and S. Flamerz, Appl. Catal., 1987, 33, 219 CrossRef CAS.
- A. La Cinestra, P. Patrono, M. L. Berardelli, P. Galli, C. Ferragina and M. A. Massucci, J. Catal., 1987, 103, 346 CrossRef CAS.
- M. Richter and G. Ohlman, Appl. Catal., 1988, 36, 81 CrossRef CAS.
- A. K. Ladavos, P. N. Trikalitis and P. J. Pomonis, J. Mol. Catal. A: Chem., 1996, 106, 241 CrossRef CAS.
- W. R. Patterson and J. J. Rooney, J. Catal., 1994, 146, 310 CrossRef CAS.
| This journal is © the Owner Societies 2003 |
