Scintillation-based potassium signalling using 2,5-diphenyloxazole-tagged aza-18-crown-6†
Bruce
Clapham‡
and
Andrew J.
Sutherland
*
Chemical Engineering & Applied Chemistry, Aston University, Aston Triangle, Birmingham, UK B4 7ET. E-mail: a.j.sutherland@aston.ac.uk; Fax: +44(0)121 359 4094; Tel: +44(0)121 359 3621
First published on 28th November 2002
Abstract
In the presence of ionising radiation, an aza-18-crown-6 molecule covalently attached to a 2,5-diphenyloxazole (PPO) moiety scintillates weakly, addition of potassium ions results in enhanced levels of scintillation, the degree of scintillation reflecting the concentration of the potassium ions.
One of the goals of supramolecular chemistry is the construction of molecular devices that perform specific tasks.1 Much interest has been directed towards the design and synthesis of fluorescence based molecular sensors and switches.2 Typically, these devices are composed of a host molecule covalently attached to a fluorophore. Complexation of an appropriate guest, for example a metal cation, by the host perturbs the photoemissive characteristics of the fluorophore and thus host–guest complexation may be detected and quantified via fluorescence emission spectroscopy. Scintillation is a photoemissive process closely related to fluorescence whereby energy in the form of a radioactive decay is converted into light energy via interaction with a scintillant molecule. Light emitted in this manner may be detected and quantified in an appropriate scintillation counter. As part of our on-going research programme involving scintillation, we were intrigued by the possibility of utilising scintillation counting to detect and quantify molecular recognition events. In this communication, we describe a scintillant-containing aza-crown ether that can be used as a sensor to detect and quantify the concentration of potassium ions in situvia scintillation counting. To our knowledge, this is the first instance of a molecular device that converts energy in the form of a radioactive emission into light energy as a consequence of a molecular recognition event.
2,5-Diphenyloxazole 1 (PPO) scintillates efficiently in the presence of ionising radiation and is the most active ingredient in most commercial scintillation cocktails. Recently, we reported the synthesis and scintillating efficiencies of a series of 4-functionalised-2,5-diphenyoxazoles.3 This study provided information about the optimum covalent linkage for attaching a scintillant reporter molecule to a receptor molecule. Specifically, 4-aminomethyl-2,5-diphenyoxazole 2 was found to have an extremely high scintillation counting efficiency, 91% as efficient as 2,5-diphenyloxazole 1 at 10mM in toluene. Since amines can quench fluorescence4 this was a suprising result and prompted us to investigate scintillation counting efficiency of scintillant tagged crown ether molecules that contained an amine linkage inbetween the receptor and reporter portion of the molecule.
Scintillant-tagged aza-18-crown-6 4 was synthesised by reaction of aza-18-crown-6 with 4-bromomethyl-2,5-diphenyloxazole 33 under standard conditions§ (Scheme 1). The scintillating properties of this novel receptor were subsequently evaluated using our standard scintillation efficiency assay.3,5 Despite the earlier findings that suggested an amine linkage between the scintillant and receptor molecule could potentially be tolerated, scintillant-tagged aza-crown 4 gave only a very low (12%) scintillating counting efficiency. We ascribed this disappointing finding to the electrons on the nitrogen atom of the aza-crown unit being more accessible to quench scintillation than in the related 4-aminomethyl-2,5-diphenyoxazole 2.
 | ||
| Scheme 1 Synthesis of scintillant-tagged aza-18-crown-6 4. | ||
It is well known that the binding cavity of aza-18-crown-6 is of the appropriate dimensions to bind potassium ions6 and so the scintillation efficiency assay of 4 was repeated in the presence of potassium ions. Significantly, higher counts per minutes (cpm) were obtained from this system, suggesting that complexation of potassium by the aza-crown was in someway enabling the scintillant reporter tag to function more efficiently. In an attempt to investigate this finding further and to quantify the effect observed, a more in-depth study of this phenomenon was undertaken.
Equal aliquots of a 10mM toluene solution of scintillant tagged aza-18-crown-6 4 were incubated with equal aliquots of [14C]hexadecane and increasing amounts of potassium thiocyanate (0–10 mM). Monitoring in a scintillation counter enabled the cpm obtained from the scintillant tag to be correlated with the amount of potassium thiocyanate present. In two separate control experiments, 4 was replaced in the assay procedure by (i) 2,5-diphenyloxazole 1 (10 mM in toluene) and (ii) a mixture of 2,5-diphenyloxazole 1 and aza-18-crown-6 (both components having a concentration of 10 mM). The three separate assays were performed under identical conditions and each assay was repeated in quadruplicate. The graph in Fig. 1, which relates scintillation counting with the concentration of potassium thiocyanate, summarises our findings.
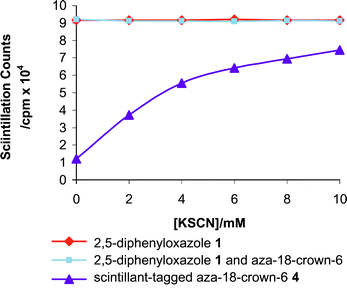 | ||
| Fig. 1 Graph relating scintillation counts to the concentration of potassium thiocyanate added to (i) 2,5-diphenyoxazole 1, (ii) 2,5-diphenyloxazole 1 and aza-18-crown-6 and (iii) scintillant-tagged aza-18-crown-6 4. | ||
Clearly, the concentration of potassium thiocyanate present in each of the two control experiments has no effect on scintillation counting efficiency. However, the presence of a covalent link between the scintillant reporter tag and the receptor molecule leads to a pronounced increase in the scintillation counting efficiency upon the addition of potassium ions. Moreover, the increase in scintillation counting efficiency relates directly to the concentration of potassium thiocyanate present and thus scintillant-tagged receptor 4 may be used as a sensor for potassium ions.
There are many examples of guest-induced ‘off–on’ switching of amine containing fluorophore-tagged receptor molecules that respond to cation binding in this way.7 Some of these systems also respond in a similar off–on fashion when the nitrogen atom(s) that they contain are protonated.4a,8 The pH dependent, fluorescent responses observed for these systems occur because of the amine lone electron pair(s). These electron pair(s) quench fluorescence by a process known as photoinduced electron transfer (PET).2c,9 Thus, when the amine becomes protonated, the lone electron pair is not available for quenching and the emission signal of the system is restored. We believed our system, based upon scintillation, would behave in the same manner. Accordingly, the scintillation counting efficiency of scintillant-tagged aza-18-crown-6 4 was evaluated in the presence of increasing amounts of trifluoroacetic acid (TFA). As observed with potassium ions, the scintillating efficiency of 4 increased with the addition of increasing amounts of acid (see ESI†).
From the experiments outlined above, it is apparent that the low scintillation counting efficiency observed for 4 in the absence of potassium ions/protons results from scintillation quenching by the lone electron pair of the nitrogen within the aza-l 8-crown-6 moiety. This quenching can be overcome by the complexation of potassium ions by the aza-crown ether or by protonation of the aza-crown nitrogen atom. Since PET is both distance and orientation dependent, it is most likely that unprotonated primary amine 2 is still able to scintillate efficiently as a result of possessing greater conformational freedom than scintillant-tagged aza-18-crown-6 4. An alternative explanation may be simply that quenching is related to the different basicities of the nitrogen atoms in primary amine 2 and tertiary amine 4, respectively.
In conclusion, we have synthesised scintillant-tagged aza-18-crown-6 4 and demonstrated that this molecule may be used as a sensor for both potassium ions and protons in toluene solutions. This is a significant finding, since we believe it constitutes the first example of a new class of molecular sensor which utilises scintillation rather than fluorescence to detect molecular recognition events. To our knowledge, the only previously-described molecular sensor that utilised radioactivity, was a cyclodextrin-based excimer system that degraded upon exposure to strongly ionising radiation.10 Subsequent changes in the fluorescence emission spectrum enabled quantitation of the radiation dose. We have also established that the aza-crown nitrogen atom’s lone electron pair is responsible for the scintillation quenching observed for the scintillant-tagged aza-18-crown-6 4 in the absence of potassium ions/protons.
Notes and references
- Reviewed in: Structure and Bonding: Molecular Machines and Motors, ed. J.-P. Sauvage, Springer-Verlag, Heidelberg, 2001, p. 99 Search PubMed, and references therein.
- (a) K. Rurack and U. Resch-Genger, Chem. Soc. Rev., 2002, 31, 116 RSC; (b) A. P. de Silva, D. B. Fox, T. S. Moody and S. M. Weir, Trends Biotechnol., 2001, 19, 29 CrossRef CAS; (c) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef; (d) L. Fabbrizzi, M. Licchelli, G. Rabaioli and A. Taglietti, Coord. Chem. Rev., 2000, 205, 85 CrossRef CAS.
- B. Clapham and A. J. Sutherland, Tetrahedron Lett., 1997, 38, 9061 CrossRef CAS.
- (a) A. P. de Silva and S. A. de Silva, Chem. Commun., 1986, 1709 RSC; (b) G. S. Cox, N. J. Turro, N. C. Yang and M. J. Chen, J. Am. Chem. Soc., 1984, 106, 422 CrossRef.
- (a) B. Clapham and A. J. Sutherland, Tetrahedron Lett., 2000, 41, 2253 CrossRef CAS; (b) B. Clapham and A. J. Sutherland, Tetrahedron Lett., 2000, 41, 2257 CrossRef CAS.
- G. W. Gokel, Crown Ethers and Cryptands,, ed. J. F. Stoddart, vol. 3 in Monographs in Supramolecular Chemistry, The Royal Society of Chemistry, London, 1991, ch. 3 Search PubMed.
- Reviewed in: A. P. de Silva, D. B. Fox, A. J. M. Huxley and T. S. Moody, Coord. Chem. Rev., 2000, 205, 41 Search PubMed.
- (a) S. A. de Silva, B. Amorelli, D. C. Isidor, K. C. Loo, K. E. Crooker and Y. E. Pena, Chem. Commun., 2002, 1360 RSC; (b) S. A. de Silva, A. Zavaleta, D. E. Baron, O. Allam, E. V. Isidor, N. Kashimura and J. M. Percarpio, Tetrahedron Lett., 1997, 38, 2237 CrossRef CAS; (c) A. P. de Silva, H. Q. N. Gunaratne and C. P. McCoy, Chem. Commun., 1996, 2399 RSC.
- R. A. Bissell, A. P. de Silva, H. Q. N. Gunaratne, P. L. M. Lynch, G. E. M. Maguire, C. P. McCoy and K. R. A. S. Sandanayake, Top. Curr. Chem., 1993, 168, 223 Search PubMed.
- C. J. Broan, Chem. Commun., 1996, 699 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: titration of scintillant-tagged 4 against TFA. See http://www.rsc.org/suppdata/cc/b2/b209337e/ |
| ‡ Current address: Department of Chemistry, BCC582, The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, CA 92037, USA. |
§ Synthesis of 2,5-diphenyloxazole-N-ethyl-aza-[18-C-6]: to a solution of aza-18-C-6 (243 mg, 0.92 mmol) in acetonitrile (5 cm3) was added 4-bromomethyl-2,5-diphenyloxazole (304 mg, 0.97 mmol) and potassium carbonate (382 mg, 2.77 mmol). The resultant mixture was heated to reflux for 3 h, cooled to room temperature, diluted with hydrochloric acid (2 M, 25 cm3) and extracted with diethyl ether (25 cm3). The aqueous layer was made alkaline by the portion-wise addition of potassium carbonate followed by the addition of an aqueous solution of sodium hydroxide (4 M, a few drops) and then extracted with ethyl acetate (2 × 30 cm3). The combined organic extracts were dried over anhydrous magnesium sulfate and concentrated under reduced pressure to give 2,5-diphenyloxazole-N-methyl-aza-[18-C-6] 4 (392 mg, 82%) as a colourless oil; νmax (thin film)/cm−1 3056, 2865, 1594, 1551; δH (270 MHz; CDCl3) 2.95 (4 H, t, J 6), 3.61–3.69 (20 H, m), 3.89 (2 H, s), 7.44–7.47 (6 H, m), 7.87–7.90 (2 H, m), 8.08–8.10 (2 H, m); δC (67.8 MHz) 51.8, 53.8, 69.7, 70.3, 70.8, 126.3, 126.4, 128.0, 128.6, 128.65, 130.1, 135.2, 148.0, 159.2; m/z (EI) 497.2645 [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H+. C28H37N2O6 requires 497.2652], 519.2474 [M H+. C28H37N2O6 requires 497.2652], 519.2474 [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na+. C28H36N2O6Na requires 519.2471]. Na+. C28H36N2O6Na requires 519.2471]. |
| This journal is © The Royal Society of Chemistry 2003 |

