Lactide polymerization by well-defined calcium coordination complexes: comparisons with related magnesium and zinc chemistry†
Malcolm H.
Chisholm
*,
Judith
Gallucci
and
Khamphee
Phomphrai
Department of Chemistry, The Ohio State University, 100 W. 18th Ave, Columbus, Ohio 43210, USA. E-mail: chisholm@chemistry.ohio-state.edu
First published on 5th December 2002
Abstract
Amide and alkoxide coordination complexes of calcium supported by β-diiminato and bulky trispyrazolylborate complexes are reported together with their activity in lactide ring-opening polymerization; some are amongst the most active systems discovered to date.
Well defined coordination complexes1 have been found to be effective in initiating the ring-opening polymerization (ROP) of lactides to give polylactides, PLAs, which are an important emerging class of environmentally friendly and biocompatible polymers, readily available from an inexpensive renewable resource.2 The β-diiminate (BDI) ligand CH[CMeN(2,6-iPr2C6H3)]2, L, has proved a particularly effective spectator ligand when attached to Mg2+, Zn2+ and Sn2+ in complexes of the form LMX where X = an amide or alkoxide which acts as an initiator in ring-opening.3–7 As with any polymer having commercial interest in the biomedical filed, it is important that the metal be biological benign and that any residual catalyst not impart undesirous properties such as color to an otherwise colorless polymer. In this regard, calcium is an attractive metal. In comparison to magnesium and zinc, calcium is significantly larger and its organometallic and coordination chemistry is notably different and much less well developed than those of magnesium and zinc. We describe here our initial foray aimed at preparing complexes of the type LCaX where X = an amide or alkoxide capable of initiating ROP of lactide. These initial results are both extremely promising with regard to the potential utility of well-defined calcium coordination complexes for the ROP of lactide and illuminating with respect to the differences in the coordination chemistry of the elements Ca, Mg, and Zn.
The bulky β-diiminate ligand LH reacts with KN(SiMe3)2 (2 equiv.) in THF and subsequently with CaI2 to yield the complex LCa[N(SiMe3)2]·THF, 1 (see Fig. 1).‡ However, based on variable temperature 1H NMR data, this compound is either dimeric at low temperatures in toluene-d8 or exists in equilibrium with other calcium species. Compound 1 polymerizes rac-lactide (200∶1 lactide∶catalyst) in THF at room temperature to 90% completion in 2 h giving atactic polylactide. When 2 equiv. of LH is used in the above preparation, the bis-β-diiminato complexes, CaL2 (see Fig. 2)‡ is obtained.
![An ORTEP drawing of LCa[N(SiMe3)2]·THF, 1, with thermal ellipsoids drawn at the 50% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and bond angles (°): Ca–N1 2.352(1), Ca–N2 2.370(1), Ca–N3 2.313(1), Ca–O 2.359(1); N1–Ca–N2 81.14(5), N1–Ca–N3 124.79(5), N1–Ca–O 113.79(5), N2–Ca–N3 136.78(5), N2–Ca–O 91.25(5), N3–Ca–O 104.88(5).](/image/article/2003/CC/b208679d/b208679d-f1.gif) | ||
| Fig. 1 An ORTEP drawing of LCa[N(SiMe3)2]·THF, 1, with thermal ellipsoids drawn at the 50% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and bond angles (°): Ca–N1 2.352(1), Ca–N2 2.370(1), Ca–N3 2.313(1), Ca–O 2.359(1); N1–Ca–N2 81.14(5), N1–Ca–N3 124.79(5), N1–Ca–O 113.79(5), N2–Ca–N3 136.78(5), N2–Ca–O 91.25(5), N3–Ca–O 104.88(5). | ||
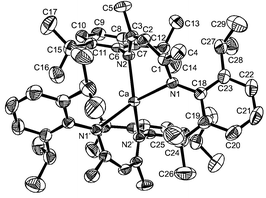 | ||
| Fig. 2 An ORTEP drawing of CaL2 with thermal ellipsoids drawn at the 50% probability level. Selected bond lengths (Å) and bond angles (°): Ca–N1 2.384(1), Ca–N2 2.377(1); N1–Ca–N2 83.08(4), N1–Ca–N1′ 126.12(6), N1–Ca–N2′ 123.26(4), N2–Ca–N1′ 123.27(4). | ||
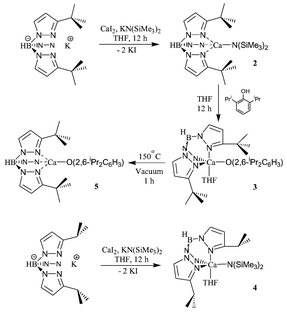
From reactions employing the more bulky trispyrazolylborate ligands, we have been able to isolate discrete four- and five-coordinate complexes of Ca2+(Scheme 1, Fig. 3 and Fig. 4). The use of the 3-tert-butylpyrazole derivate allows the isolation of the four-coordinate amide [HB(3-tBupz)3]CaN(SiMe3)2, 2, and the five-coordinate aryl oxide [η3-HB(3-tBupz)3]Ca(O-2,6-iPr2C6H3)·THF, 3. There is little doubt that the coordination geometry of the latter compound parallels that of the 3-isopropylpyrazole derivative, [η3-HB(3-iPrpz)3]CaN(SiMe3)2·THF, 4, shown in Fig. 3. Even though the quality of a single crystal X-ray structure of compound 4 is poor, we can state that compound 4 has a five-coordinate Ca2+ center with THF at an axial position. A similar five-coordinate structure was found for Mg2+ in [η3-HB(3-Phpz)3]MgEt·THF.5 The THF in compound 3 can be removed by heating at 150 °C under vacuum giving a four-coordinate complex [η3-HB(3-tBupz)3]Ca(O-2,6-iPr2C6H3), 5,‡ in quantitative yield (see Fig. 4). The [HB(3-tBupz)3]Ca amide and aryl oxide compounds initiate and sustain the ring-opening polymerization of lactides. Indeed, compounds 2 and 5 are remarkably active for lactide polymerization, and both show stereoselectivity in the polymerization of rac-lactide to >90% heterotactic PLA (isi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sis) in THF at room temperature.7,8 The polymerization approaches 90% conversion in 1 min ([LA]0/[2]0 = 200, Mn = 37.8 kg mol−1, PDI = 1.74 and [LA]0/[5]0 = 200, Mn = 40.1 kg mol−1, PDI = 1.68) (see Fig. 5). Interestingly, the less bulky [η3-HB(3-iPrpz)3]CaN(SiMe3)2·THF shows no stereoselectivity under the same condition ([LA]0/[4]0 = 200, 97% conversion in 1 min, Mn = 64.0 kg mol−1, PDI = 1.61).
sis) in THF at room temperature.7,8 The polymerization approaches 90% conversion in 1 min ([LA]0/[2]0 = 200, Mn = 37.8 kg mol−1, PDI = 1.74 and [LA]0/[5]0 = 200, Mn = 40.1 kg mol−1, PDI = 1.68) (see Fig. 5). Interestingly, the less bulky [η3-HB(3-iPrpz)3]CaN(SiMe3)2·THF shows no stereoselectivity under the same condition ([LA]0/[4]0 = 200, 97% conversion in 1 min, Mn = 64.0 kg mol−1, PDI = 1.61).
 | ||
| Scheme 1 | ||
![An ORTEP drawing of [η3-HB(3-iPrpz)3]CaN(SiMe3)2·THF, 4, with thermal ellipsoids drawn at the 30% probability level.](/image/article/2003/CC/b208679d/b208679d-f3.gif) | ||
| Fig. 3 An ORTEP drawing of [η3-HB(3-iPrpz)3]CaN(SiMe3)2·THF, 4, with thermal ellipsoids drawn at the 30% probability level. | ||
![An ORTEP drawing of [η3-HB(3-tBupz)3]Ca(O-2,6-iPr2C6H3), 5, with thermal ellipsoids drawn at the 50% probability level. Selected bond lengths (Å) and bond angles (°): Ca–N1 2.437(1), Ca–N3 2.426(1), Ca–N5 2.412(1), Ca–O 2.106(1); N1–Ca–N3 84.22(4), N1–Ca–N5 85.43(4), N1–Ca–O 120.48(4), N3–Ca–N5 79.84(4), N3–Ca–O 132.31(4), N5–Ca–O 136.79(4), Ca–O–C22 174.4(1).](/image/article/2003/CC/b208679d/b208679d-f4.gif) | ||
| Fig. 4 An ORTEP drawing of [η3-HB(3-tBupz)3]Ca(O-2,6-iPr2C6H3), 5, with thermal ellipsoids drawn at the 50% probability level. Selected bond lengths (Å) and bond angles (°): Ca–N1 2.437(1), Ca–N3 2.426(1), Ca–N5 2.412(1), Ca–O 2.106(1); N1–Ca–N3 84.22(4), N1–Ca–N5 85.43(4), N1–Ca–O 120.48(4), N3–Ca–N5 79.84(4), N3–Ca–O 132.31(4), N5–Ca–O 136.79(4), Ca–O–C22 174.4(1). | ||
.](/image/article/2003/CC/b208679d/b208679d-f5.gif) | ||
| Fig. 5 1H NMR spectra (CDCl3, 400 MHz) of the homodecoupled CH resonance of poly(rac-lactide) prepared in THF using [η3-HB(3-tBupz)3](O-2,6-iPr2C6H3). | ||
In examining the relative reactivities of [HB(3-tBupz)3]M complexes we note that under identical conditions 100 equiv. of LA are converted to >90% PLA at room temperature in 1 min for M = Ca, 1 h for M = Mg and 6 days for M = Zn which clearly defines the reactivity order Ca > Mg > Zn. Moreover, in competitive experiments where mixtures of (BDI)MN(SiMe3)2 were allowed to react with 1 equiv. of LA, we observed the same trend. When calcium and magnesium were both present effectively only the calcium amide reacted while for mixtures of magnesium and zinc, the magnesium reacted to the exclusion of the zinc amide which remained after consumption of LA.
In conclusion, we have discovered some interesting new coordination compounds of calcium and found that some of these are amongst the most active of all known coordination compounds in the ring-opening polymerization of lactides. Some fascinating comparisons within the coordination chemistry of Ca2+, Mg2+ and Zn2+ are also to be noted. Further studies are in progress.
We thank the Department of Energy, Office of Basic Energy Science, Chemistry Division for support of this work. K. P. acknowledges the Institute for the Promotion of Teaching Science and Technology (IPST), Thailand, for an opportunity to work on this project.
Notes and references
- B. J. O′Keefe, M. A. Hillmyer and W. B. Tolman, J. Chem. Soc., Dalton Trans., 2001, 15, 2215 RSC.
- S. K. Ritter, Chem. Eng. News, 2002, 26 Search PubMed.
- M. Cheng, A. B. Attygalle, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 1999, 121, 11583 CrossRef CAS.
- M. H. Chisholm, J. Gallucci and K. Phomphrai, Inorg. Chem., 2002, 41, 2785 CrossRef CAS.
- M. H. Chisholm, N. W. Eilerts, J. C. Huffman, S. S. Iyer and M. Pacold and K. Phomphrai, J. Am. Chem. Soc., 2000, 122, 11845 CrossRef CAS.
- A. P. Dove, V. C. Gibson, E. L. Marshall, A. J. P. White and D. J. Williams, Chem. Commun., 2001, 283 RSC.
- B. M. Chamberlain, M. Cheng, D. R. Moore, T. M. Ovitt, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 3229 CrossRef CAS.
- H. R. Kricheldorf, C. Boettcher and K. U. Tonnes, Polymer, 1992, 33, 2817 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: syntheses and characterization of compounds 1–5 and CaL2. See http://www.rsc.org/suppdata/cc/b2/b208679d/ |
‡ Crystal data: LCa[N(SiMe3)2]·THF, 1: C42H74CaN3OSi2, M = 733.30, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 11.912(1), b = 13.222(1), c = 16.888(1) Å, α = 70.150(1), β = 80.422(1), γ = 63.507(1)°, V = 2238.64(3) Å3, T = 200 K, Z = 2, μ = 0.226 mm−1, 47522 collected reflections, 10257 independent reflections, R indices (all data) R = 0.0633, wR = 0.1362. CCDC 192310.CaL2: C58H82CaN4, M = 437.68, monoclinic, C2/c, a = 19.513(2), b = 12.527(1), c = 22.147(1) Å, β = 100.280(1)°, V = 5326.53(8) Å3, T = 200 K, Z = 4, μ = 0.157 mm−1, 46450 collected reflections, 6114 independent reflections, R indices (all data) R = 0.0635, wR = 0.1317. CCDC 192311.[η3-HB(3-tBupz)3]Ca(O-2,6-iPr2C6H3), 5: C33H51BCaN6O, M = 598.69, monoclinic, P21/c, a = 15.177(1), b = 11.737(1), c = 20.885(2) Å, β = 110.690(1)°, V = 3480.34(5) Å3, T = 200 K, Z = 4, μ = 0.214 mm−1, 60025 collected reflections, 7975 independent reflections, R indices (all data) R = 0.0542, wR = 0.1102. CCDC 192309.See http://www.rsc.org/suppdata/cc/b2/b208679d/ for crystallographic data in CIF or other electronic format. , a = 11.912(1), b = 13.222(1), c = 16.888(1) Å, α = 70.150(1), β = 80.422(1), γ = 63.507(1)°, V = 2238.64(3) Å3, T = 200 K, Z = 2, μ = 0.226 mm−1, 47522 collected reflections, 10257 independent reflections, R indices (all data) R = 0.0633, wR = 0.1362. CCDC 192310.CaL2: C58H82CaN4, M = 437.68, monoclinic, C2/c, a = 19.513(2), b = 12.527(1), c = 22.147(1) Å, β = 100.280(1)°, V = 5326.53(8) Å3, T = 200 K, Z = 4, μ = 0.157 mm−1, 46450 collected reflections, 6114 independent reflections, R indices (all data) R = 0.0635, wR = 0.1317. CCDC 192311.[η3-HB(3-tBupz)3]Ca(O-2,6-iPr2C6H3), 5: C33H51BCaN6O, M = 598.69, monoclinic, P21/c, a = 15.177(1), b = 11.737(1), c = 20.885(2) Å, β = 110.690(1)°, V = 3480.34(5) Å3, T = 200 K, Z = 4, μ = 0.214 mm−1, 60025 collected reflections, 7975 independent reflections, R indices (all data) R = 0.0542, wR = 0.1102. CCDC 192309.See http://www.rsc.org/suppdata/cc/b2/b208679d/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2003 |
