Transparent thin films and monoliths synthesized from fullerene doped mesoporous silica: evidence for embedded monodispersed C60†
Selvaraj
Subbiah
and
Robert
Mokaya
*
School of Chemistry, University of Nottingham, University Park, Nottingham, UK NG7 2RD. E-mail: r.mokaya@nottingham.ac.uk; Fax: +44 115 9513562; Tel: +44 115 8466174
First published on 28th November 2002
Abstract
Thin films and monoliths of mesostructured silica containing embedded monodispersed molecules of C60 may be prepared via a sol–gel route in which the C60 is added during the synthesis or via post-synthesis adsorption; evidence from diffuse reflectance spectroscopy suggests that the embedded C60 exist predominantly in monomeric form.
In recent years, fullerenes (C60, C70) and their derivatives have been subjected to intense research investigations for a wide range of potential applications in materials science.1 In particular fullerenes show spectrally broad Reverse Saturable Absorption (RSA), covering the entire visible region, and can therefore be used as optical limiting materials.2 Their optical limiting properties have been studied in both solid and liquid phases.3,4 When comparing the results of C60 in solution and in solid form (as crystalline films), it becomes clear that the interaction between neighbouring fullerene molecules largely determines the de-excitation dynamics and consequently the optical limiting properties.5 In other words the photophysical properties of formation of the triplet state of C60 are extremely dependent on the environment of the molecule.6 It has been shown that when C60 molecules aggregate the yield of formation of the triplet state considerably decreases, thus compromising the optical limiting capability of such aggregate molecules.7
In order to avoid aggregation and undesirable interactions between C60 molecules, we have studied the dispersion of fullerene molecules within the pores of mesoporous molecular sieves (MMS). MMS are generally synthesized in the form of powders.8 However, mesoporous films and monoliths are more usable, especially in membrane-based separation or novel optical/electronic applications such as in optical limiting. Here we report our attempts to disperse fullerene molecules within the pores of MMS films and monoliths via a surfactant mediated preparation route or via post-synthesis adsorption. It is hoped that once embedded, the C60 molecules are better protected from environmental influences that are detrimental to their optical limiting properties.9 Previous reports on the interaction between C60 and silica hosts provide limited information on the nature of the incorporated C60;10–14 in most cases only showing evidence for the incorporation of C60 in the form of aggregates or clusters.
In a typical synthesis procedure, TEOS, HCl and water were added to a round bottomed flask containing a mixture of 1.0 g of cetyltrimethylammonium bromide (CTAB, Aldrich), ethanol and a toluene solution of C60 ( 0.7 mM). The solution consisting of a single phase (with a molar ratio of CTAB/TEOS = 0.2) was refluxed at 80 °C for 2 h. The reaction mixture was then concentrated by the quick evaporation of the solvent using a rotary evaporator in a vacuum at 50 °C. The resulting high viscosity liquid was poured on a Petri dish and dried at room temperature for 12 h to yield the transparent thin films or monoliths. In order to vary the fullerene content, different volumes of toluene/C60 solution were added during the synthesis.
The XRD patterns of the as-synthesized C60 containing transparent silica mesophases shown in Fig. 1 clearly indicate that ordered materials are formed. The pattern for the as-synthesised sample shows sharp (100 and 200) peaks at d = 3.5 nm and 1.8 nm respectively. There is no evidence of residual CTAB surfactant peaks which are known to occur at d = 2.6 and 1.3 nm. The films or monoliths varied in thickness from 5 to 200 μm. After calcination at 550 °C for 6 h in air, the films/monoliths (which maintained their transparency) had XRD patterns similar to the as-synthesised materials (Fig. 1). Calcination did however cause a shift of the observed peaks to 2.9 nm and 1.5 nm (i.e., a decrease of ca. 0.6 nm in the basal spacing). The XRD patterns of the films are consistent with the formation of a hexagonal mesoporous silica framework. The fact that only h00 (100, 200) low angle reflections are observed implies that the pore channels preferentially run parallel to the surface of the film.15 The XRD pattern of the ground powder samples (not shown) had the expected (100), (110), (200), (210) peaks from diffraction planes that are typically seen in randomly oriented powder preparations of hexagonal mesoporous silica.16 The intense peaks of pure C60 that normally occur at 2θ = 10, 17, and 21° were not detected. The absence of these peaks is an indication of the occlusion of fullerene in the pores of the MMS. Adsorption of C60 onto the calcined mesoporous films/monoliths had no effect on the XRD patterns.
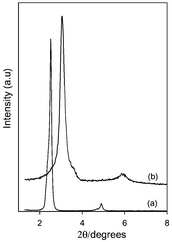 | ||
| Fig. 1 XRD patterns of C60 containing mesoporous film before (a) and after (b) calcination. | ||
The UV-Vis absorption spectrum of C60 is known to be sensitive to environmental effects (solvent, aggregation) and can be used to probe aggregation. The diffuse reflectance spectrum of C60 embedded in the MMS was found to be generally broader than that of solid C60 (i.e., C60 in KBr) or C60 in solution (toluene) as shown in Fig. 2. C60 in toluene, which exists predominantly in monomeric form, has a characteristic sharp peak (spike) at 397 nm and multiple bands which appear at 537–541, 560–569, 592–598 and 621 nm.17 It is well known that a sharp peak (spike) at ca. 400 nm is an indication of C60 in monomeric form while a band at ca. 450 nm is attributed to the formation of aggregates.6,18,19 The spectra obtained for C60 embedded in the MMS mesophase exhibit features similar to that of C60 in toluene, i.e., they both have the sharp peak (spike) at ca. 400 nm (i.e., 397 nm for C60 in toluene and 405 nm for C60 in MMS) and very weak absorption at ca. 470 nm. The presence of the characteristic spike, slightly red shifted to ca. 405 nm, is clear evidence of the presence of C60 monodispersed in the silica hosts.18,19 It is noteworthy that the spike is absent for solid C60 (i.e., C60 in KBr, Fig. 2b) which is known to exist in aggregates. We note that the broad low intensity band in the 450–500 nm region (observed for both C60/MMS and C60/toluene) whilst indicating some limited aggregation also further illustrates the similarity between the two systems. The similarity between the C60/MMS and C60/toluene systems is a strong indication that, even after incorporation in the mesoporous matrix, C60 exists predominantly as monomeric species without significant formation of aggregates or clusters. This contrasts with what has previously been observed when C60 is embedded in inorganic hosts.11–14
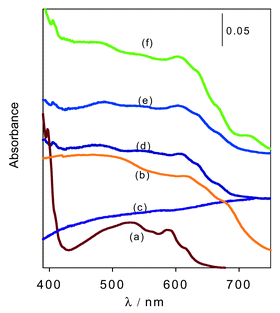 | ||
| Fig. 2 Diffuse reflectance spectra of C60 embedded in mesoporous molecular sieve (MMS) films. The spectra of the MMS film, C60 in KBr and UV-Vis spectra of C60 in toluene are included for comparison. (a) C60 in toluene, (b) C60 in KBr, (c) MMS in the absence of C60, (d) C60/SiO2 (mol ratio = 1.5 × 10−4), (e) C60/SiO2 (mol ratio = 2.0 × 10−4), (f) C60/SiO2 (mol ratio = 2.0 × 10−4, 20 h contact time). | ||
The diffuse reflectance spectra of C60 embedded (via post-synthesis adsorption) in calcined MMS films are shown in Fig. 3 for various C60/SiO2 mol ratios. For typical C60/SiO2 mol ratios (up to 1.5 ×10−3, spectrum a) the embedded C60 exists predominantly in monodispersed form, that is, the spectrum shows the characteristic spike near 400 nm and a low intensity band at ca. 440 nm. However at higher C60 content we observed a gradual change from monodispersion to aggregation as shown in Fig. 3. The spike near 400 nm, which is an indication of monodispersion, is just discernible at a C60/SiO2 mol ratio of 2.4 ×10−3 (spectrum b) and disappears at a C60/SiO2 mol ratio of 4.07 ×10−3 (spectrum c). This is accompanied by a more prominent band at ca. 440 nm. This semi-quantitative analysis of the diffuse reflectance spectra confirms that the spike near 400 nm is observed when the C60 is predominantly monodispersed and is consistent with previous reports.18,19
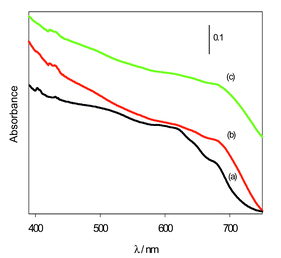 | ||
| Fig. 3 Diffuse reflectance spectra of C60 embedded in calcined mesoporous molecular sieve (MMS) films at various C60/SiO2 mol ratios: (a) 1.22 × 10−3, (b) 2.42 × 10−3 and (c) 4.07 × 10−3. | ||
All the C60 containing thin films/monoliths also had an absorption band at ca. 330 nm (see ESI†) which was also present for C60 in toluene. This band, assigned to the transition of the 31T1u excited state of C60, has previously been used as evidence for the incorporation of C60 into solid hosts.14,18 As expected, Fig. 2 and 3 show a red shift in the spectra of C60 in MMS films compared to C60 in toluene. The red shift is usually observed when C60 is immobilized in a polar environment.20,21 We also note that the considerable absorption in the visible region (420 to 700 nm) suggests that the symmetry of the fullerenes is somewhat distorted as would be expected for C60 embedded in a solid host.20,21 The diffuse reflectance spectra therefore point to a scenario where the MMS embedded C60 exists predominantly (but perhaps not exclusively) in a monomeric form similar to that of C60 in toluene. The presence of monodispersed C60 in a transparent host matrix is likely to lead to solid state materials with enhanced optical limiting properties.
This work was funded by the EPSRC.
Notes and references
- G. Brusatin and R. Signorini, J. Mater. Chem., 2002, 12, 1964 RSC; Y-P. Sun and J. E. Riggs, Int. Rev. Phys. Chem., 1999, 18, 43 CrossRef CAS.
- P. Innocenzi and G. Brusatin, Chem. Mater., 2001, 13, 3126 CrossRef CAS; G. Brusatin and P. Innocenzi, J. Sol–Gel Sci. Technol., 2001, 22, 189 Search PubMed.
- V. Klimov, L. Smilowitz, H. Wang, M. Grigorova, J. M. Robinson, A. Koskelo, B. R. Mattes, F. Wudl and D. W. McBranch, Chem. Intermed., 1997, 23, 587 Search PubMed.
- S. Couris, E. Koudoumas, A. A. Ruth and S. Leach, J. Phys. B: At. Mol. Opt. Phys., 1995, 28, 4537 Search PubMed.
- L. Smilowitz, D. McBranch, V. Klimov, J. M. Robinson, M. Grigorova, B . J. Weyer, A. Koskelo, B. R. Mattes, H. Wang and F. Wudl, Synth. Met., 1997, 84, 931 CrossRef CAS.
- R. V. Bensasson, E. Bienvenue, M. Dellinger, S. Leach and P. Seta, J. Phys. Chem., 1994, 98, 3492 CrossRef CAS.
- J. M. Janot, E. Bienvenue, P. Seta, R. V. Bensasson, C. Tome, R. F. Enes, J. A. S. Calvaleivo, S. Leach, X. Camps and A. Hirsch, J. Chem. Soc., Perkin Trans. 2, 2000, 301 RSC.
- J. Y. Ying, C. P. Mehnert and M. S. Wong, Angew. Chem., Int. Ed., 1999, 38, 56 CrossRef CAS.
- J. Schell, D. Felder, J. F. Nierengarten, J.-L. Rehspringer, R. Levy and B. Honerlace, J. Sol–Gel Sci. Technol., 2001, 22, 225 Search PubMed.
- F. Rachdi, L. Hajji, C. Goze, D. J. Jones, P. M. Torres and J. Roziere, Solid State Commun., 1996, 100, 237 CrossRef CAS; A. Govindaraj, M. Nath and M. Eswaramoorthy, Chem. Phys. Lett., 2000, 317, 35 CrossRef CAS.
- S. Dai, R. N. Compton, J. P. Young and G. Mamantov, J. Am. Ceram. Soc., 1992, 75, 2865 Search PubMed.
- T. W. Zerda, A. Brodka and J. Coffer, J. Non-Cryst. Solids, 1994, 168, 33 Search PubMed.
- M. Maggini, G. Scorrano, M. Prato, G. Brusatin, P. Innocenzi, M. Guglielmi, A. Renier, R. Signorini, M. Meneghetti and R. Bozio, Adv. Mater., 1995, 7, 404 CrossRef CAS.
- I. Hasegawa and S. Nonomura, J. Sol–Gel Sci. Technol., 2000, 19, 297 Search PubMed.
- H. Yang, N. Coombs, I. Sokolov and G. A. Ozin, Nature (London), 1996, 381, 589 CrossRef CAS.
- C. T. Kresge, M. Leonowicz, W. J. Roth, J. C. Vartuli and J. C. Beck, Nature (London), 1992, 359, 710 CrossRef CAS.
- S. K. Lin, L. L. Shin, K. M. Chien, T. Y. Luh and T. I. Lin, J. Phys. Chem., 1995, 99, 105 CrossRef CAS.
- A. Beeby, J. Eastoe and R. K. Heenan, J. Chem. Soc., Chem. Commun., 1994, 173 RSC.
- J. Eastoe, E. R. Crooks, A. Beeby and R. K. Heenan, Chem. Phy. Lett., 1995, 571 Search PubMed.
- I. Hasegawa, K. Shibusa, S. Kobayashi, S. Nonomura and S. Nitta, Chem. Lett., 1997, 995 CrossRef CAS.
- O. H. Kwon, H. Yoo and O. J. Jang, Eur. Phys. J. D, 2002, 18, 69 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: diffuse reflectance spectra of C60 embedded in mesoporous molecular sieve films. See http://www.rsc.org/suppdata/cc/b2/b208615h/ |
| This journal is © The Royal Society of Chemistry 2003 |
