Capture and detection of a quencher labeled oligonucleotide by poly(phenylene ethynylene) particles†
Joong
Ho Moon
,
Robert
Deans
,
Elizabeth
Krueger
and
Lawrence F.
Hancock
*
Nomadics, Inc., 61 Rogers Street, Cambridge, MA 02142, USA. E-mail: lhancock@nomadics.com; Fax: 1 617 441 8874; Tel: 1 617441 8871
First published on 28th November 2002
Abstract
Fluorescence quenching of poly(phenylene ethynylene) (PPE) particles by a Cy-5 labeled oligonucleotide is 2 orders of magnitude more sensitive than direct excitation of the Cy-5 fluorophore.
We report a new PPE that forms sub-micron particles using phase inversion fabrication. The dispersed PPE particles are then used to capture a Cy-5 labeled oligonucleotide with high efficiency and detect them via fluorescence quenching. The combined effects of enhanced fluorescence quenching by the conjugated PPE and strong adsorption of the Cy-5 oligonucleotide to the PPE particle provide a remarkably sensitive means to detect the Cy-5 labeled oligonucleotide. This example shows that detection of the labeled oligonucleotide by fluorescence quenching is at least 2 orders of magnitude more sensitive than detection of the Cy-5 fluorophore by direct excitation. This suggests that the subject PPE may be used to improve the sensitivity of microarray-based gene expression methodologies where Cy-5 labeled cDNA targets are widely used.
Luminescent conjugated polymers show the remarkable ability to enhance fluorescence quenching effects.1,2 Swager showed enhanced quenching of PPEs by electron accepting analytes, most notably trinitrotoluene (TNT).1b Whitten and coworkers extended the principal to show amplification using a water soluble, anionic poly(phenylene vinylene).2 In both examples, detection relies on strong binding of the quencher and polymeric fluorphore and on exciton migration. The intrinsic mobility of an exciton to migrate within the extended π-orbital system of the luminescent polymer is the root of the observed enhanced quenching. The net effect of exciton mobility is that chromophores, distant from a quenching analyte, will absorb energy and then transport it to the analyte. Thus, a single bound quencher will affect numerous chromophores of the conjugated polymer. This effect is more pronounced in the solid state where exciton migration becomes a three-dimensional effect with exciton transfer between chains nearly as important as intrachain energy transfer. Static binding also plays an important role in detection in these systems.3 Static binding holds the quencher in close contact with the conjugated polymer, thereby allowing it to continually shunt excitons to the ground state.
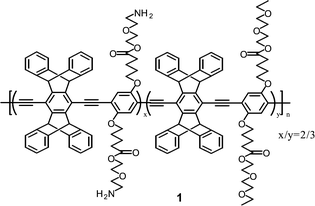
This work uses a modified, pentiptycene-based PPE similar to those first described by Yang and Swager.1b Polymer 1 is a copolymer formed by the polycondensation of a bis-ethynyl pentiptycene monomer and two substituted diiodophenyl monomers via palladium cross-coupling (see ESI).†1H NMR indicates a 2/3 ratio of the amine and diethylene oxide containing repeat units, respectively.
Polymer 1 forms dispersed particles in aqueous solutions by phase inversion methods. In the present example, 1 is dissolved in DMSO (0.1 mg ml−1) and is added to an excess volume of an aqueous SSPE4 buffer. Polymer 1 is soluble in DMSO but insoluble in water, hence as DMSO is dispersed into the aqueous phase, the polymer precipitates to form particles. The aqueous dispersion is optically clear, however, its absorbance and emission spectra (see ESI) are reminiscent of a spin cast film consistent with the formation of dispersed, solid particles.
Transmission electron microscopy confirms that 1 forms dispersed particles in aqueous solutions, Fig. 1. Electron micrographs revealed nearly spherical particles with diameters of 500–800 nm. Dynamic light scattering results show a mean particle size of 400–500 nm for similarly prepared particles. Close inspection of individual particles reveals that the particles have a uniform porous structure. The particle formation mechanism appears to be phase inversion precipitation. One important variable in particle fabrication is the chemical structure of the polymer, especially the nature and density of the hydrophilic side groups. In this example, the pendant protonated amine and the short chain non-ionic diethylene oxide moiety stabilize the surface of the forming particle and hinder aggregation and precipitation. Others have reported nanoparticle formation with styrene/carboxystyrene copolymers5 and various block copolymers.6 Other important variables in the particle formation process include concentration of the polymer solution, the solvent and diluent used, as well as pH, salt concentration and mixing parameters. These are currently being explored in our lab.
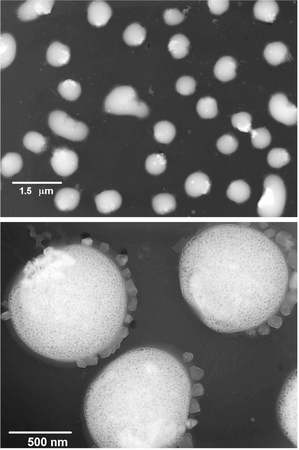 | ||
| Fig. 1 Transmission electron micrographs of particles formed by phase inversion precipitation of 1 dissolved in DMSO at 0.1 mg ml−1 and precipitated into a 100 fold excess of 1 × SSPE aqueous buffer. | ||
Pentiptycene based PPEs are unique in that a relatively large measure of their native, solution-state fluorescence is preserved when the materials are condensed to the solid state.1b This allows three dimensional energy migration and supports enhanced quenching effects.7 Thus the fabrication of PPE particles provides a practical advantage in that the solid-state fluorescence amplification of these materials is translated into a high surface area, dispersed, aqueous format suitable for bioassays.
The capture and detection of a labeled oligonucleotide by an aqueous dispersion of 1 provides an example of the utility of these particles for biosensing. Fig. 2 summarizes experiments where particles of 1 dispersed in 1 × SSPE are titrated with a Cy-5 labeled oligonucleotide. While Cy-5 is noted more as a fluorophore, here the most prominent effect is an instantaneous quenching of the particle emission. This is consistent with previously reported quenching effects by Cy-5 dyes.8 Similar quenching effects are observed with oligonucleotides labeled with other known quenchers including BHQ-1, dabcyl and dinitrophenol. The main plot of Fig. 2 shows the emission of particles of 1 excited at 415 nm. The primary emission of the polymer particles centers on 463 nm. The plot goes on to show emission traces where the Cy-5 oligonucleotide has been added to the particle dispersion over 4 decades of Cy-5 oligonucleotide concentration (10−10 to 10−7 M in Cy-5 oligo). The plot clearly shows that the fluorescence of the polymer particles is progressively quenched from 7% to 72%. The Cy-5 label is responsible for quenching the particles. In a control experiment, no quenching is observed with an even greater concentration of unlabeled oligonucleotide (10−6 M). It is also unlikely that the observed quenching is due to attenuation of the excitation light by the adsorbed Cy-5 oligonucleotide as its extinction coefficient at 415 nm is approximately 1400 cm−1 M−1.
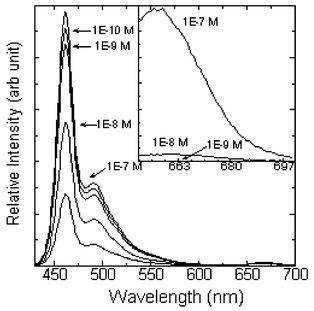 | ||
| Fig. 2 Fluorescence (λex = 415 nm) of 1 dispersed as particles in 1 × SSPE and equivalent concentrations of 1 after addition of Cy-5 labeled oligonucleotide. The inset shows direct excitation of the Cy-5 label (λex = 635 nm) for a subset of samples. | ||
The Cy-5 labeled oligonucleotide provides an opportunity to directly compare the sensitivity of fluorescence quenching with that of direct excitation of the Cy-5 label. The inset in Fig. 2 shows the emission from the Cy-5 label for a subset of oligonucleotide concentrations. Emission from the Cy-5 label (λex = 635 nm) is not detected under our experimental conditions below 10−8 M Cy-5 oligonucleotide. In contrast, the same sample displays 44% quenching of the polymer particle fluorescence. As the concentration is decreased further, fluorescence quenching of the PPE particles is readily observed, whereas direct excitation of the Cy-5 produced no detectable emission. Thus we observe at least a 2 order of magnitude lower detection threshold with the fluorescence quenching measurement as compared to a measurement by direct excitation of the Cy-5 fluorophore.
Stern–Volmer analysis (see ESI) of the fluorescence quenching experiment can be used to estimate the efficiency of the interaction between the Cy-5 oligonucleotide and the PPE particle. The Stern–Volmer constant is 8.8 × 107 M−1. Note that this exceptionally high KS-V is a composite reflecting enhanced quenching due to exciton migration in the conjugated polymer and strong adsorption of the quencher bearing oligonucleotide onto the particle surface.
The value of the reported work is in the translation of luminescent conjugated polymers and their enhanced quenching effects, into a physical format that is generally applicable in biological assays. In particular, these materials may prove valuable for fluorescence detection in microfluidic systems and in homogeneous high throughput screening systems. The work also demonstrates that the detection limit of the fluorescence quenching measurement is at least 2 orders of magnitude lower than a fluorescence measurement using direct excitation of the Cy-5 label. This result may lead directly to improved sensitivity in microarray-based gene expression methodologies where Cy-5 labeled cDNA targets are widely used.
The authors thank the National Science Foundation (SBIR Grant 0128052) for partial support of this work. The authors also thank Professor N. Kotov and Dr Z. Tang, Oklahoma State University for assistance obtaining the TEMs.
Notes and references
- (a) Z. Qin and T. M. Swager, J. Am. Chem. Soc., 1995, 117, 7017 CrossRef; (b) J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864 CrossRef CAS; (c) D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS; (d) T. M. Swager, Acc. Chem. Res., 1998, 31, 201 CrossRef CAS.
- (a) L. Chen, D. W. McBranch, H.-L. Wang, R. Helgeson, F. Wudl and D. G. Whitten, Proc. Natl. Acad. Sci. USA, 1999, 96, 12287 CrossRef CAS; (b) L. Chen, D. McBranch, R. Wang and D. G. Whitten, Chem. Phys. Lett., 2000, 330, 27 CrossRef CAS; (c) L. Chen, S. Xu, D. McBranch and D. Whitten, J. Am. Chem. Soc., 2000, 122, 9302 CrossRef CAS.
- Examples of quenching enhancement on dispersed solid supports, see: (a) R. M. Jones, L. Lu, R. Helgeson, T. S. Bergstedt, D. W. McBranch and D. G. Whitten, Proc. Natl. Acad. Sci. USA, 2001, 98, 14769 CrossRef CAS; (b) Y.-K. Gong, T. Miyamoto, K. Nakashima and S. Hashimoto, J. Phys. Chem. B, 2000, 104, 5772 CrossRef CAS.
- SSPE (saline, sodium phosphate, EDTA) is a buffer commonly employed for nucleic acid hybridization. 1 × SSPE contains 50 mM NaHPO4, 150 mM NaCl and 5 mM EDTA.
- (a) G. Zhang, A. Niu, S. Peng, M. Jiang, Y. Tu, M. Li and C. Wu, Acc. Chem. Res., 2001, 34, 249 CrossRef CAS; (b) M. Li, M. Jiang, L. Zhu and C. Wu, Macromolecules, 1997, 30, 2201 CrossRef CAS.
- (a) B. Lotz and A. J. Kovacs, Kolloid Z. Z. Polym., 1966, 209, 97 Search PubMed; (b) B. Lotz, A. J. Kovacs, G. A. Bassett and A. Keller, Kolloid Z. Z. Polym., 1966, 209, 115 Search PubMed; (c) K. A. Cogan and A. P. Gast, Macromolecules, 1990, 23, 745 CrossRef CAS; (d) A. P. Gast, P. K. Vinson and K. A. Cogean-Farinas, Macromolecules, 1993, 26, 1774 CrossRef CAS; (e) C. Wu, J. Fu and Y. Zhao, Macromolecules, 2000, 33, 9040 CrossRef CAS; (f) D. Portinha, J. Belleney, L. Bouteiller, S. Pensec, N. Spassky and C. Chassenieux, Macromolecules, 2002, 35, 1484 CrossRef CAS.
- Comparing with an isolated PPE chain in a good solvent, which shows mainly one-dimensional energy transfer through the polymer backbone, the three-dimensional energy migration in the particles through space results in enhanced fluorescence quenching. For examples of vectorial energy transfer, see: (a) J. Kim, D. T. McQuade, A. Rose, Z. Zhu and T. M. Swager, J. Am. Chem. Soc., 2001, 123, 11488 CrossRef CAS; (b) D. T. McQuade, A. H. Hegedus and T. M. Swager, J. Am. Chem. Soc., 2000, 122, 12389 CrossRef CAS; (c) I. A. Levitsky, J. Kim and T. M. Swager, J. Am. Chem. Soc., 1999, 121, 1466 CrossRef CAS.
- (a) D. Doizi and J. C. Mialocq, J. Phys. Chem., 1987, 91, 3524 CrossRef CAS; (b) H. Killesreiter, Z. Naturforsch., 1979, 34a, 737 Search PubMed; (c) W. West, in Dye Sensitizsation, ed. W. F. Berg, U. Mazzucato, H. Meier and G. Semerano, Focal Press, London, UK, 1970, p. 105 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: synthesis and characterization of monomers and polymer 1, absorbance and emission spectra of 1, Stern–Volmer analysis. See http://www.rsc.org/suppdata/cc/b2/b207186j/ |
| This journal is © The Royal Society of Chemistry 2003 |