Inverse Wittig reaction of oxaphosphetenes formed by the [2+2] cycloaddition of arylphosphine oxides and dimethyl acetylenedicarboxylate (DMAD)
György
Keglevich
*a,
Henrietta
Forintos
a,
Tamás
Körtvélyesi
b and
László
Tőke
c
aDepartment of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
bDepartment of Physical Chemistry, University of Szeged, 6701 Szeged, Hungary
cResearch Group of the Hungarian Academy of Sciences at the Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
First published on 22nd November 2001
Abstract
The intermediate oxaphosphetenes 2 formed by the novel cycloaddition of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of arylphosphine oxides 1 and the acetylene moiety of DMAD are stabilised by an inverse Wittig reaction to afford the corresponding stabilised phosphonium ylide 3.
O group of arylphosphine oxides 1 and the acetylene moiety of DMAD are stabilised by an inverse Wittig reaction to afford the corresponding stabilised phosphonium ylide 3.
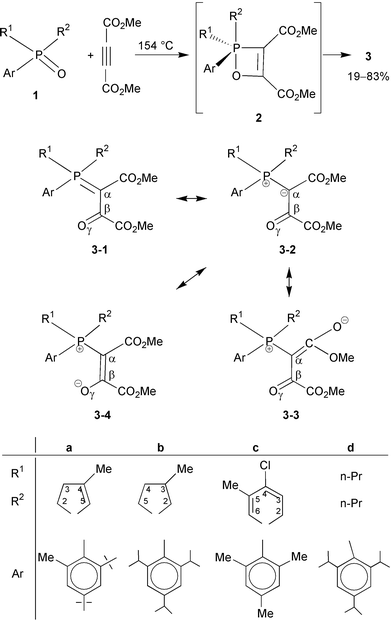
We have recently reported that a series of 2,4,6-trialkylphenylphosphine oxides underwent a novel [2+2] cycloaddition with DMAD.1 On the basis of analogies2 and spectroscopic data, we thought first that the products were the oxaphosphetenes themselves formed by the cycloaddition of the P
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the acetylene moiety.3 Later on, it was suggested, however, by the results of semiempirical calculations that the oxaphosphetene structure contains considerable ring strain.4 HF/6-31G* ab initio calculations supported the conclusion that the four-membered species can be only intermediates.5 The products of the reaction of the cyclic or acyclic trialkylphenylphosphine oxides 1a–d with DMAD are now shown to be the stabilised ylides 3a–d
formed by the ring opening of intermediates 2a–d (Scheme 1). In an extension of the reactants investigated, compounds 3a–d, obtained in variable yields after chromatography, were characterised by 31P, 13C and 1H NMR, as well as by their IR and mass spectroscopic data, including HRMS. The 16.7–43.6 range of the δP values unambiguously supported the phosphonium salt character of the product 3, and hence the involvement of the resonance structures 3-2, 3-3 and 3-4. It is, however, clear from the IR spectra refined by derivation that a keto carbonyl moiety (at νC
O group and the acetylene moiety.3 Later on, it was suggested, however, by the results of semiempirical calculations that the oxaphosphetene structure contains considerable ring strain.4 HF/6-31G* ab initio calculations supported the conclusion that the four-membered species can be only intermediates.5 The products of the reaction of the cyclic or acyclic trialkylphenylphosphine oxides 1a–d with DMAD are now shown to be the stabilised ylides 3a–d
formed by the ring opening of intermediates 2a–d (Scheme 1). In an extension of the reactants investigated, compounds 3a–d, obtained in variable yields after chromatography, were characterised by 31P, 13C and 1H NMR, as well as by their IR and mass spectroscopic data, including HRMS. The 16.7–43.6 range of the δP values unambiguously supported the phosphonium salt character of the product 3, and hence the involvement of the resonance structures 3-2, 3-3 and 3-4. It is, however, clear from the IR spectra refined by derivation that a keto carbonyl moiety (at νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
≈
1663 cm−1) and two ester groups (at νC
O
≈
1663 cm−1) and two ester groups (at νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
≈ 1713 and 1761 cm−1) are present in product 3 thus justifying the resonance structure 3-1.
O
≈ 1713 and 1761 cm−1) are present in product 3 thus justifying the resonance structure 3-1.
 | ||
| Scheme 1 | ||
The optimised structure of product 3c determined by HF/6-31G* calculation is shown in Fig. 1. It is worth noting that the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and the C
C and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oγ bonds are slightly elongated (1.690 and 1.234 Å, respectively,) while the Cα–Cβ bond is somewhat shortened (1.436 Å). The distance between the P1 and Oγ atoms is 2.87 Å.
Oγ bonds are slightly elongated (1.690 and 1.234 Å, respectively,) while the Cα–Cβ bond is somewhat shortened (1.436 Å). The distance between the P1 and Oγ atoms is 2.87 Å.
 | ||
| Fig. 1 Perspective view of 3c. Selected bond lengths/Å: P1–Cα 1.690, Cα–Cβ 1.436, Cβ–Oγ 1.234, P1–C6 1.768, C6–C5 1.340, C5–C4 1.463, C4–C3 1.344, C3–C2 1.471, C2–P1 1.834, P1–C1′ 1.827. | ||
The only criterion of the novel reaction is that the phosphorus atom should bear an electron-donating trialkylphenyl substituent. The presence of a 2,4,6-triisopropylphenyl group is the optimum in this respect; with 2,4-di-tert-butyl-6-methylphenyl, there is increased steric hindrance, while with 2,4,6-trimethylphenyl, the electron-releasing ability is lower, resulting in a decrease in the efficiency of the reaction.
Careful observation shows that the transformation 2→3 can be regarded as an intramolecular inverse Wittig reaction, as it formally involves the rupture of the P–O bond and the formation of a P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and a C
C and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond. This kind of reaction has never been observed before since it would not be possible to generate a phosphetene intermediate via a Wittig reaction of an ylide with a carbonyl compound. Kano and Kawashima have described, however, a similar azaphosphete→phosphorane conversion.6
O double bond. This kind of reaction has never been observed before since it would not be possible to generate a phosphetene intermediate via a Wittig reaction of an ylide with a carbonyl compound. Kano and Kawashima have described, however, a similar azaphosphete→phosphorane conversion.6
It can be concluded that the novel reaction of trialkylphenylphosphine oxides (1) and DMAD, which is especially efficient with cyclic phosphine oxides (1a–c), gives an entry to transient oxaphosphetenes (2) that are stabilised by a retro Wittig type reaction to furnish the corresponding stabilised phosphonium ylides (3).
Experimental
General method for the synthesis of compounds 3a–d
A mixture of the phosphine oxide 1a–d (2.0 mmol) and DMAD (4.0 ml, 32.5 mmol) was kept at 154 °C for 8–14 days in a sealed tube. The excess of the reagent was removed in vacuo. The residue thus obtained was purified by repeated column chromatography (3% methanol in chloroform, silica gel) to give the products 3a–d as oils.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.1 (J
= 14.4, C
O), 168.1 (J
= 14.4, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 183.6 (J
= 7.0, Cβ) (* assignments may be exchanged);
IR (film) 1669, 1714, 1763 cm−1; (M + H)+found
= 461.2445, C26H38O5P requires 461.2457.
O), 183.6 (J
= 7.0, Cβ) (* assignments may be exchanged);
IR (film) 1669, 1714, 1763 cm−1; (M + H)+found
= 461.2445, C26H38O5P requires 461.2457.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 167.9 (J
= 14.6, C
O), 167.9 (J
= 14.6, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 183.1 (J
= 6.2, Cβ); IR (film) 1671,
1715, 1754 cm−1; (M + H)+found
= 463.2633, C26H40O5P requires 463.2613.
O), 183.1 (J
= 6.2, Cβ); IR (film) 1671,
1715, 1754 cm−1; (M + H)+found
= 463.2633, C26H40O5P requires 463.2613.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 167.7 (J
= 15.8, C
O), 167.7 (J
= 15.8, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 182.9 (J
= 6.2, Cβ); IR (film) 1648, 1711, 1767 cm−1; (M + H)+found
= 423.1060, C21H25ClO5P
requires 423.1128 for the 35Cl isotope.
O), 182.9 (J
= 6.2, Cβ); IR (film) 1648, 1711, 1767 cm−1; (M + H)+found
= 423.1060, C21H25ClO5P
requires 423.1128 for the 35Cl isotope.
Acknowledgements
Support from the Hungarian Scientific Research Fund (OTKA, Grant No. T029039) and the Ministry of Higher Education (FKFP, Grant No. 363/1999) is gratefully acknowledged. Gy. K. is grateful for the advice of Dr Igor Shevchenko (Institute of Bioorganic Chemistry and Petrochemistry, Kiev, Ukraine).References
- Gy. Keglevich, H. Forintos, Á. Szöllősy and L. Tőke, Chem. Commun., 1999, 1423 RSC.
- T. Kawashima, T. Iijma, H. Kikuchi and R. Okazaki, Phosphorus, Sulfur Silicon Relat. Elem., 1999, 144, 149 Search PubMed.
- Gy. Keglevich, H. Forintos, Gy. M. Keserű, L. Hegedűs and L. Tőke, Tetrahedron, 2000, 56, 4823 CrossRef CAS.
- Gy. Keglevich, T. Körtvélyesi, H. Forintos, A. Tamás, K. Ludányi, V. Izvekov and L. Tőke, Tetrahedron Lett., 2001, 42, 4417 CrossRef CAS.
- Gaussian 98, Revision A. 6, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Clioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle and J. A. Pople, Gaussian, Inc., Pittsburgh PA, 1998.
- N. Kano, J.-H. Xing, A. Kikuchi and T. Kawashima, Heteroat. Chem., 2001, 12, 282 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |
