Hot off the press
Robert A.
Hill
* and
Marie Claire
Parker
Department of Chemistry, Glasgow University, Glasgow, UK G12 8QQ. E-mail: bobh@chem.gla.ac.uk, m.parker@chem.gla.ac.uk
First published on 9th January 2002
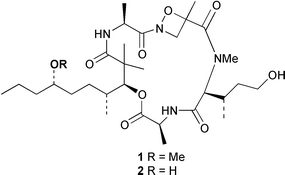
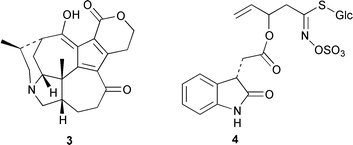
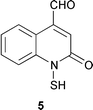
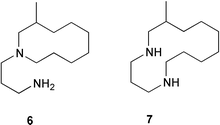
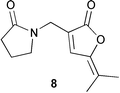
Two anti-inflammatory cyclic depsipeptides halipeptins A 1 and B 2 have been isolated from a marine sponge of the Haliclona species (L. Gomez-Paloma and co-workers, J. Am. Chem. Soc., 2001, 123, 10870). The halipeptins contain a 17-membered ring with five residues; two are L-alanine while the remaining three residues are novel. The daphnicyclidins, such as A 3, are a group of hexa- and pentacyclic alkaloids from two Daphniphyllum species (J. Kobayashi et al., J. Am. Chem. Soc., 2001, 123, 11402). A group of indole glucosinolates, including glucoisatisin 4, has been isolated from woad, Isatis tinctoria (N. Fabre and co-workers, Tetrahedron Lett., 2001, 42, 9015).The unusual N-mercapto-4-formylcarbostyril 5 is an antibiotic produced by Pseudomonas fluorescens (W. Fakhouri et al., Phytochemistry, 2001, 58, 1297). The structure of the marine alkaloid haliclorensin from Haliclona tulearensis has been revised from 6 to 7 (W. Steglich and co-workers, Tetrahedron, 2001, 57, 9973). The new structure was confirmed by synthesis. The novel alkaloid uncinine 8 has been isolated from Artabotrys uncinatus (Y.-C. Wu and co-workers, J. Nat. Prod., 2001, 64, 1157).
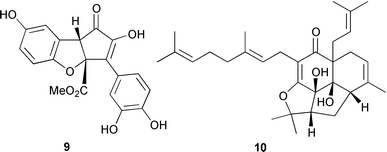
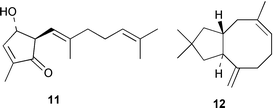
Suillusin 9, from the mushroom Suillus granulatus, has a highly substituted 1H-cyclopenta[b]benzofuran (I.-D. Yoo and co-workers, J. Nat. Prod., 2001, 64, 1230). A dome-shaped polyprenylated tetracyclic phloroglucinol derivative, erectone A 10, has been identified from the Chinese herb Hypericum erectum (T.-Y. An et al., Tetrahedron Lett., 2002, 43, 163). Litseaverticillol A 11, with a new sesquiterpenoid framework, is an anti-HIV agent from Litsea verticillata (H.-J. Zhang et al., Tetrahedron Lett., 2001, 42, 8587). The structure of the sesquiterpenoid asterisca-3(15),6-diene 12 has been revised on the basis of synthesis (G. Mehta and J. D. Umarye, Tetrahedron Lett., 2001, 42, 8101). Asterisca-3(15),6-diene 12 was previously thought to have a cis-ring fusion.
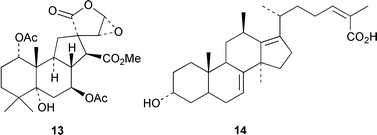
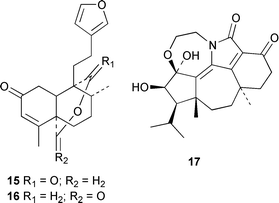
Spirocaesalmin 13, from Caesalpinia minax, is a novel rearranged vouacapane diterpenoid (T. C. W. Mak and co-workers, J. Chem. Soc., Perkin Trans. 1, 2001, 2920). The first example of an 18(13→12)-abeo-lanostane, ananosic acid 14, has been isolated from Kadsura ananosma (Y.-G. Chen et al., Phytochemistry, 2001, 58, 1277). Synthetic studies have established that the structure of the clerodane diterpenoid sacacarin should be revised from the 19,20-olide 15 to the 20,19-olide 16 (R. B. Grossman and R. M. Rasne, Org. Lett., 2001, 3, 4027). The endophytic fungus CR115 contains a highly diverse family of diterpenoids, the guanacastepenes, including guanacastepene H 17 (J. Clardy and co-workers, J. Am. Chem. Soc., 2001, 123, 9900).
Secoisolariciresinol diglucoside 18 is a natural cancer chemopreventive agent found as glutaryl esters from flax seed, Linum usitissimum. Feeding studies have been used to establish the biosynthetic pathway to secoisolariciresinol diglucoside 18 as outlined in Scheme 1 (N. G. Lewis and co-workers, J. Nat. Prod., 2001, 64, 1388). The origin of the carbon skeleton of pyridoxamine in two yeasts, Saccharomyces cerevisiae and Candida utilis, has been shown by labelling studies to be substantially different to that found in Escherichia coli (I. D. Spenser and co-workers, J. Am. Chem. Soc., 2001, 123, 11353).
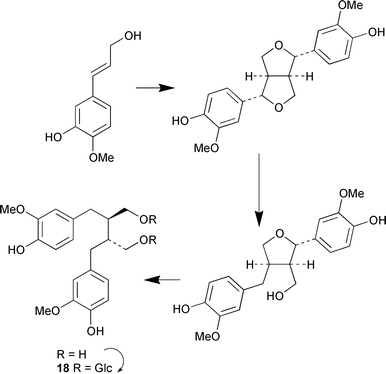 | ||
| Scheme 1 | ||
Feeding studies using Streptomyces cattleya have indicated that fluoroacetaldehyde 19 is formed from a C3 glycolytic intermediate rather than a C2 amino acid metabolite (Scheme 2) (D. O'Hagan and co-workers, J. Chem. Soc., Perkin Trans. 1, 2001, 3100). Fluoroacetaldehyde 19 is the common precursor of fluoroacetate and 4-fluorothreonine 20. A PLP-dependent threonine transaldolase has been identified as an enzyme involved in 4-fluorothreonine 20 biosynthesis (D. O'Hagan and co-workers, Angew. Chem.,Int. Ed., 2001, 40, 4479). The authors propose a mechanism for this transformation (Scheme 3). Labelled glucose has been used to establish biosynthetic pathways for terpenoids in the liverwort Fossombronia alaskana (K. P. Adam and co-workers, Phytochemistry, 2001, 58, 1049). The results indicate that a hopane triterpenoid arises from the mevalonate pathway whereas three diterpenoids are derived from the methylerythritol phosphate pathway.
 | ||
| Scheme 2 | ||
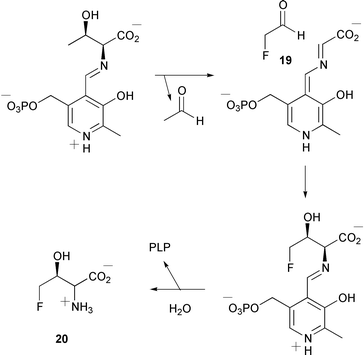 | ||
| Scheme 3 | ||
Antibiotics that target cell-wall biosynthesis and translation of RNA in bacterial ribosomes have resulted in an interest in carbohydrate mimics. T. K. Ritter and C.-H. Wong have reviewed carbohydrate-based antibiotics as an approach to tackling antibiotic resistance (Angew. Chem.,Int. Ed., 2001, 40, 3508). In a comprehensive review P. C. Wright and co-workers have described the range of natural products that are produced by marine cyanobacteria and highlight the potential of these organisms to produce biologically active natural products with novel structures (Tetrahedron, 2001, 57, 9347). B. R. O'Keefe, in a review of biologically active proteins from natural product extracts, points out that there is a wide diversity of proteinaceous material available from non-mammalian sources (J. Nat. Prod., 2001, 64, 1373).
A simple method for the oxidation of alcohols to carbonyl compounds using oxygen at room temperature has been devised (C. Galli and co-workers, Tetrahedron Lett., 2001, 42, 7551). The method uses the enzyme laccase that is a multi-copper oxidase found in the white-rot fungus Trametes villosa. The natural substrates of laccases are phenolic fragments of lignin however TEMPO 21 can also be oxidised to the oxoammonium ion 22. This ion will oxidise a variety of alcohols such as benzyl alcohols to aldehydes or ketones without over-oxidation (Scheme 4).
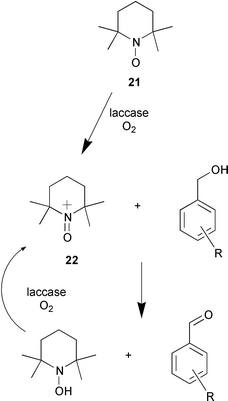 | ||
| Scheme 4 | ||
A stereoselective and chemoselective synthesis of all four stereoisomers of tert-butyl 6-chloro-3,5-dihydroxyhexanoate is described by M. Wolberg et al. (Chem. Eur. J., 2001, 7, 4562). The regio- and enantioselective single-site reduction of the β,δ-diketo esters is achieved using two enantiocomplementary biocatalysts; alcohol dehydrogenase from Lactobacillus brevis and Baker's yeast (Scheme 5). Various types of α-hydroxyketones bearing a phenyl ring were reduced with good to high enantioselectivities (Scheme 6) using the enzyme system from Yamamdazyma farinosa IFO10896 to give optically active diols with anti-Prelog selectivity (H. Ohta et al., Tetrahedron: Asymmetry, 2001, 12, 2543).
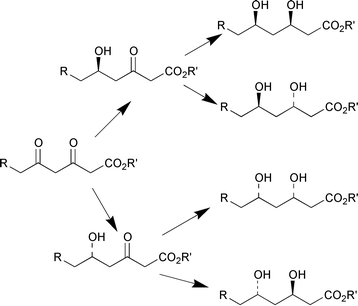 | ||
| Scheme 5 | ||
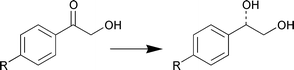 | ||
| Scheme 6 | ||
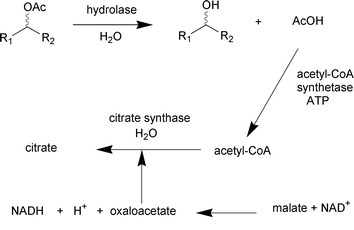
Cells of Bacillus megaterium catalysed the conversion of pyrrole to pyrrole-2-carboxylate in supercritical CO2 (Scheme 7). The yield of reaction was 12-fold greater than that under atmospheric pressure (T. Matsuda et al., Chem. Commun., 2001, 2194). A new assay format based on a coupled enzymatic conversion for high throughput-screening for the identification of active and enantioselective hydrolases is reported by U. T. Bornscheuer (Angew. Chem., Int. Ed., 2001, 40, 4201). The assay is based on the release of acetic acid as a result of lipase or esterase-catalysed hydrolysis from the corresponding chiral ester followed by a cascade of enzymatic reactions, the acetic acid reacts to form NADH which can be measured spectrophotometrically (Scheme 8). The membrane associated epoxide hydrolase from Rhodotorula glutinis was imprinted using substrates or analogues in an aqueous phase prior to co-polymerization with ethylene glycol dimethacrylate to form a bioplastic. The enantioselective conversion of (±)-1,2-epoxyoctane was reversed from a preference for (R)-1,2-epoxyoctane to (S)-1,2-epoxyoctane when the enzyme had been imprinted with (S)-1,2-epoxyoctane prior to co-polymerization (N. A. E. Kronenburg et al., J. Mol. Catal. B: Enzym., 2001, 16, 121).
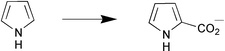 | ||
| Scheme 7 | ||
 | ||
| Scheme 8 | ||
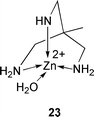
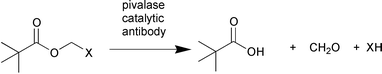
A model, composed of a Zn2+ complex of the tripodal ligand 1,1,1-tris(aminomethyl)ethane 23 catalyses the hydrolysis of a model ester showing Michaelis–Menten kinetics. The complex has been proposed by T. G. Sprigings and C. D. Hall as a simple model of the active site of the hydrolytic enzyme carbonic anhydrase (J. Chem. Soc., Perkin Trans.2, 2001, 2063). The activation of pivalate prodrugs by an esterolytic reaction using pivalase catalytic antibodies has been reported by J. L. Reymond and co-workers (Chem. Eur. J., 2001, 7, 4604). Screening of monoclonal antibody libraries generated eleven possible candidates that may be used for the activation of orally available pivaloyloxymethyl prodrugs (Scheme 9).
 | ||
| Scheme 9 | ||
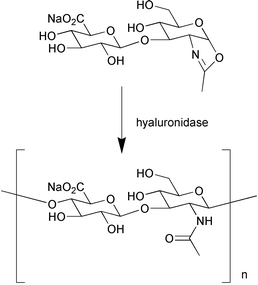
S. Kobayashi et al. (J. Am. Chem. Soc., 2001, 123, 11825) have reported a novel method for synthesising a natural heteropolysaccharide of hyaluronic acid containing both β(1→3) and β(1→4) glycosidic linkages using hyaluronidase in an enzymatic polymerisation reaction (Scheme 10). L-Selective dipeptide synthesis was achieved using a novel, inexpensive, thermophilic protease-type enzyme isolated from anaerobic Clostridium thermohydrosulfuricum. The enzyme showed broad substrate specificity and high stereoselectivity towards L-amino acids (Y. S. Yadav, Tetrahedron: Asymmetry, 2001, 12, 2505).
 | ||
| Scheme 10 | ||
P. Sebban and co-workers have reported the involvement of extended hydrogen bond networks in the co-operative function between distant centres in bacterial photosynthetic reaction centres. Their work demonstrated that proton uptake occurs by strong co-operation between structural motifs such as hydrogen-bonded networks that span the 18 Å distance between the two quinone electron acceptors (J. Tandori et al., J. Biol. Chem., 2001, 276, 45513). The development of enzyme evolution technology and in particular DNA shuffling is described by K. A. Powell and co-workers. The authors have focused on several classes of industrially relevant enzymes and their current application and limitations that require to be addressed (Angew. Chem., Int. Ed., 2001, 40, 3948). C. M. Niemeyer (Angew. Chem., Int. Ed., 2001, 40, 4128) has reviewed the current approaches emerging at the intersection of materials research, nanosciences, and molecular biotechnology. The various aspects of organic and inorganic nanoparticles, evolutionary optimised biomolecules and supramolecular structures for the formation of nanostructured architectures are discussed.
| This journal is © The Royal Society of Chemistry 2002 |