Marine natural products
D. John Faulkner
Scripps Institution of Oceanography, University of California at San Diego, La Jolla, CA 92093–0212, USA
First published on 10th December 2001
Abstract
This review covers the marine natural products literature for the year 2000 and is organized phylogenetically, with sections on marine microorganisms and phytoplankton, green algae, brown algae, red algae, sponges, coelenterates, bryozoans, molluscs, tunicates, echinoderms and miscellaneous marine organisms. There is an emphasis on new structures, stressing their biological activities, source organisms and countries of origin, and also syntheses that confirm the structures of known compounds. The review contains 869 structures and 592 references, of which 434 appeared between January and December 2000.
Covering: 2000. Previous review: 2001, 18, 1.
1 Introduction
This report is a review of the marine natural products literature for 2000. Earlier reports published in this journal cover the period from 1977 to December 1999. After many years of steady expansion in marine natural products chemistry, the number of papers published in 2000 has declined slightly when compared with 1999.1 Sponges were again the most studied of the marine organisms, closely followed by tunicates and coelenterates. The most significant increase is in the number of papers reporting studies of marine microorganisms. It is hard to say whether this is driven by advances in marine biotechnology or by the increasing difficulty of obtaining permits to collect marine invertebrates. There is currently a great interest, aided by advances in molecular genetics, in the possible production of bioactive compounds from marine invertebrates by associated microorganisms.2 Studies of the biosynthesis of marine natural products, which also bear the mark of the latest genetic techniques, are reviewed elsewhere in this journal.3 Marine natural products continue to be prime targets for synthesis although papers reporting partial and formal syntheses, which are not included in this review, outnumber the papers that record total syntheses and synthetic studies that redefine the structures of marine natural products. This report continues to emphasize the bioactivity of new marine natural products while specifically excluding biochemical and pharmacological studies of known marine natural products. The patent literature is not covered in this report. The format for this review is identical to its predecessor.A number of reviews of specific topics appeared during 2000. The major work entitled Comprehensive Natural Products Chemistry contained an extensive review of “Marine natural products and marine chemical ecology”.4 A book entitled Drugs from the Sea consisted of a number of timely reviews running the whole gamut of marine drug discovery: “Marine microorganisms and drug discovery: current status and future potential”,5 “Microalgae as a drug source”,6 “Search for biologically active substances from marine sponges”,7 “Cytotoxic substances from opisthobranch mollusks”,8 “ω-Conotoxin MVIIA: from marine snail venom to analgesic drug”,9 “KRN7000 as a new type of antitumor and immunostimulatory drug”,10 “Zoanthamines, antiosteoporotic alkaloids”,11 “Symbiotic bacteria in sponges: sources of bioactive substances”,12 “Aquacultural production of bryostatin 1 and ecteinascidin 743”,13 and “The halichondrins: chemistry, biology, supply and delivery”.14 Marine natural products are featured extensively in a review of “Naturally occurring isocyano/isothiocyanato and related compounds”.15 There have been several reviews of individual marine natural products especially those that are either in or approaching clinical trials: these include didemnin B, aplidine and ecteinascidin 743, which are reviewed in “Antitumor compounds from tunicates”,16 “The chemistry and biology of discodermolide”,17 “Chemistry and clinical biology of the bryostatins”,18 “The guanidine metabolites of Ptilocaulis spiculifer and related compounds; isolation and synthesis”,19 “Chemical constituents and their biological activities of the sponge family Aplysinellidae: A review”,20 “Marine polyether triterpenes”,21 and “The structure elucidation and biological activity of high molecular weight algal toxins: maitotoxin, prymnesins and zooxanthellatoxins”.22 In addition to the reviews listed above, an alarming number of possibly relevant reviews have been published in books and journals that were not readily available to this author: these references may be found by searching databases such as CAS Online or MarinLit.
2 Marine microorganisms and phytoplankton
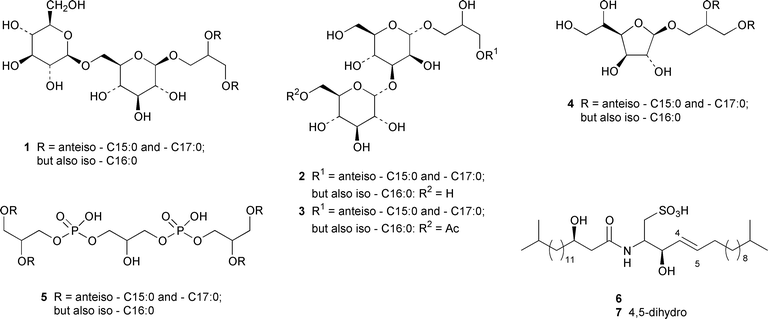
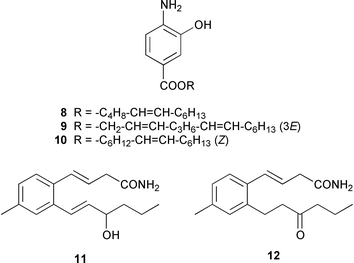
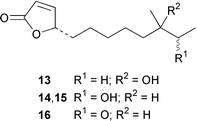
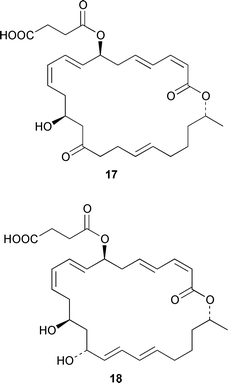
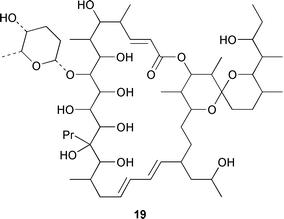
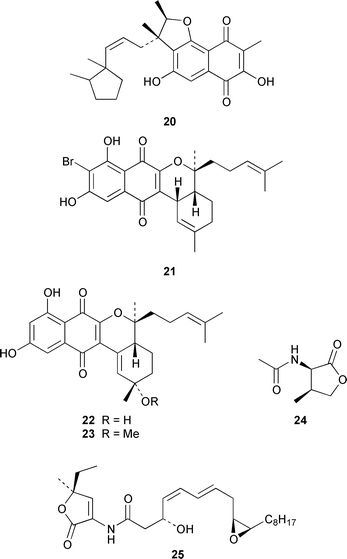
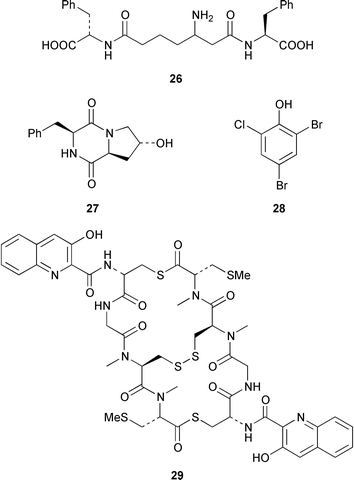
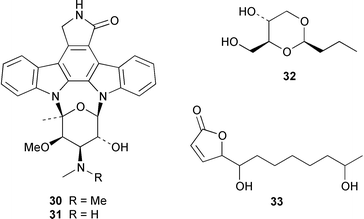
As part of an overall increase in interest in cultured marine organisms, the upswing in studies of marine fungi was particularly noticeable. Interest in marine bacteria was maintained at a significant but relatively constant level. A seawater culture of a new Microbacterium sp. isolated from the Adriatic sponge Halichondria panicea contained four cell-associated glycoglycerolipids 1–4 and a diphosphatidylglycerol 5.23 A complex mixture of gentiobosyl diglycerides was isolated from Bacillus pumilis that was associated with the ascidian Halocynthia aurantium.24 The structures of flavochristamides A 6 and B 7, which are sulfonolipids from a marine Flavobacterium sp.,25 were confirmed by total synthesis.26 The 4-amino-3-hydroxybenzoic acid derivatives, B-5354a 8, b 9 and c 10 are inhibitors of sphingosine kinase that were isolated from a Ruegeria sp. (SANK 71896) that was isolated from seawater.27 Lornemides A 11 and B 12 are mildly antimicrobial aromatic amides from a marine actinomycete isolated from beach sand in Southern Australia.28 Four new butenolides consisting of 4,10-dihydroxy-10-methyldodec-2-en-1,4-olide 13, two diastereoisomeric 4,11-dihydroxy-10-methyldodec-2-en-1,4-olides 14 and 15, and 4-hydroxy-10-methyl-11-oxododec-2-en-1,4-olide 16 were isolated from marine Streptomyces strains B 5632 and B 3497, both of which were isolated from marine sediments.29 The antibacterial activity of a Bacillus sp. (Sc026) from a marine sediment was shown to be due to the known compound macrolactin F and the new compounds 7-O-succinylmacrolactin F 17 and 7-O-succinylmacrolactin A 18.30 IB-96212 19, the stereochemistry of which remains undetermined, is a cytotoxic macrolide from a marine species of Micromonospora.31 An unidentified marine actinomycete (strain # CNH-099) from a sediment obtained near San Diego contained the cytotoxins neomarinone 20, isomarinone 21, hydroxydebromomarinone 22 and methoxydebromomarinone 23.32N-Acetyl-γ-hydroxyvaline lactone 24, obtained from a streptomycete isolated from a marine sediment from Brazil, belongs to a class of compounds implicated in quorum sensing.33 The absolute and relative stereochemistry of korormicin 25, which is a metabolite of Pseudoalteromonas sp. F-420,34 has been deduced by the synthesis of four possible diastereoisomers.35A Pseudomonas or Alteromonas sp. (DF-1), isolated from a Black Sea specimen of the sponge Dysidea fragilis, produced the tripeptide 26.36 A culture of Pseudoalteromonas luteoviolacea isolated from the surface of the Hawaiian alga Padina australis produced the diketopiperazine cyclo(L-phenylalanyl-4R-hydroxy-L-proline) 27, which stimulated antibiotic production in this strain, and 2,4-dibromo-6-chlorophenol 28, which showed activity against methicillin-resistant Staphylococcus aureus and Burkholderia cepacia.37 The stereochemistry of thiocoraline 29, which is a potent antitumor antibiotic from Micromonospora sp. L-13-ACM2–092,38 has been determined by total synthesis.39 Two new cytotoxic staurosporine derivatives, 4′-N-methyl-5′-hydroxystaurosporine 30 and 5′-hydroxystaurosporine 31, were obtained from a marine Micromonospora sp. (strain # L-31-CLCO-02) isolated from a homogenate of the sponge Clathrina coriacea from the Canary Islands.40
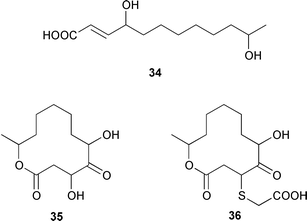
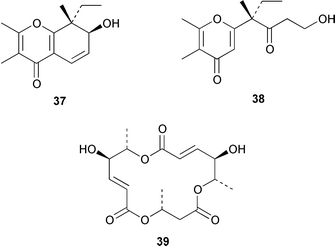
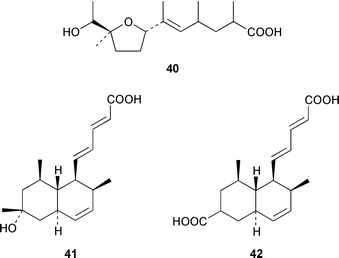
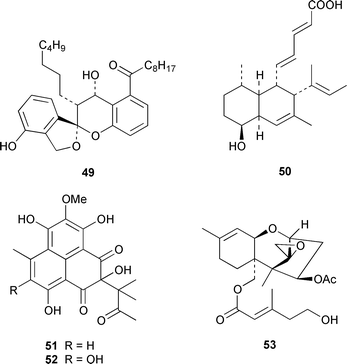
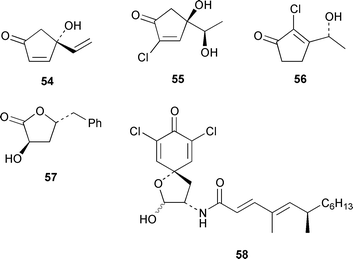
Marine derived fungi have become a dominant source of new marine metabolites. Coruscol A 32 is a relatively simple 1,3-dioxane from a Penicillium sp. cultured from the bivalve mollusc Mytilus coruscus from Okinawa.41 An unidentified fungal strain (I96S215), which was obtained from a tissue sample of an unidentified marine sponge collected in Indonesia, produced iso-cladospolide B 33 and seco-patulolide 34, together with the macrolides pandangolides 1 35 and 2 36.42 Spiciferol A 37 and butoxyl-spiciferin 38 were obtained from an isolate of Drechslera hawaiiensis obtained from an Indonesian sponge, Callyspongia aerizusa.43 (+)-Macrosphelide E 39, which is a metabolite of Periconia byssoides isolated from the gastrointestinal tract of the sea hare Aplysia kurodai,44 has been synthesized in high yield by using a chemoenzymatic reaction as a key step in the synthesis.45 A salt-water culture of Aspergillus niger (culture # 94–1212) obtained from the sponge Hyrtios proteus from the Dry Tortugas National Park, Florida, contained asperic acid 40 and several known metabolites.46 Tanzawaic acids E 41 and F 42 are additional members of that series that were isolated, together with 3,7-dimethyl-1,8-dihydroxy-6-methoxyisochroman 43, from a culture of Penicillium steckii obtained from an unidentified tunicate.47 An undescribed Microsphaeropsis sp. from the Mediterranean sponge Aplysina aerophoba contained the tyrosine kinase inhibitors 10-hydroxy-18-methoxybetaenone 44, 10-hydroxy-18-[N-(2-naphthyl)-N-phenylamino]betaenone 45, and three 1,3,6,8-tetrahydroxyanthraquinones 46–48.48 Paecilospirone 49, which is an inhibitor of microtubule assembly, was among the metabolites isolated from a Paecilomyces sp. obtained from a coral reef at Yap, Micronesia.49,50 Phomopsidin 50 was obtained from a Phomopsis sp. cultured from a fallen mangrove branch from Pohnpei, Micronesia.50 A Penicillium sp. from the Okinawan bivalve Mytilus coruscus contained sculezonones A 51 and B 52.51 A new trichothecene, verrol 4-acetate 53 was obtained from the deuteromycete Acremonium neocaledoniae, which was isolated from driftwood in New Caledonia.52 Synthetic studies of trichodenones A–C, which are cytotoxic metabolites of a strain of Trichoderma harzianum OUPS-N115 isolated from the sponge Halichondria okadai,53 revealed that trichodenone A 54 was a scalemic mixture in which the (R)-enantiomer predominated and established the absolute configurations of trichodenones B 55 and C 56.54 The absolute stereochemistry of harzialactone A 57, which was also isolated from the same strain of T. harzianum,53 was determined by total synthesis from D-glucose.55 The absolute stereostructure of gymnastatin A 58, which is a cytotoxic metabolite of Gymnasella dankialiensis,56,57 has been confirmed by total synthesis.58
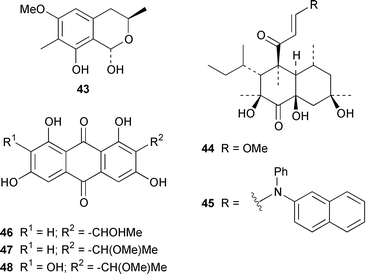
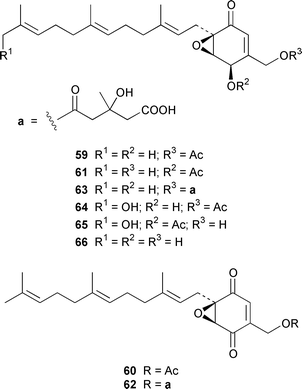
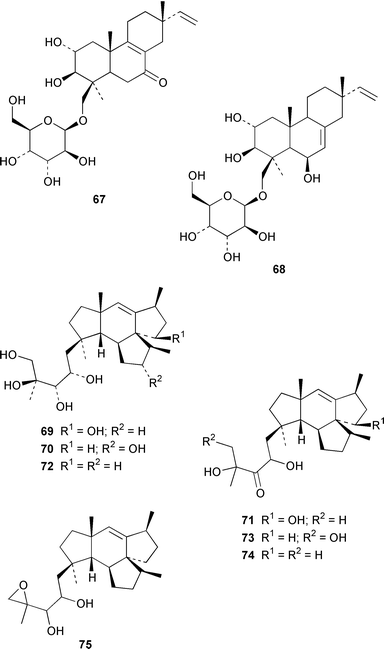
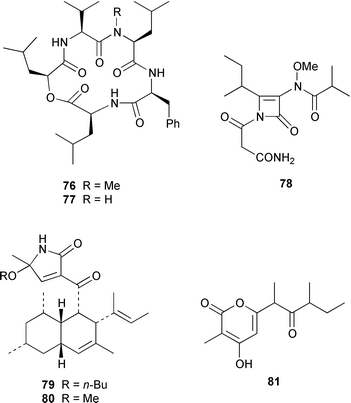
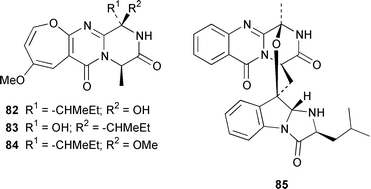
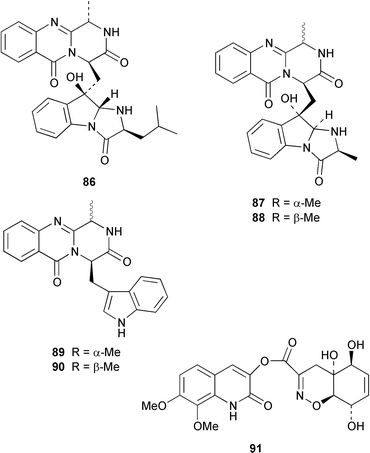
A specimen of Aspergillus niger that was cultured from a tissue homogenate of an orange Aplidium sp. tunicate produced the meroterpenoids yanuthones A–E 59–63, 1-hydroxyyanuthone A 64, 1-hydroxyyanuthone C 65 and 22-deacetylyanuthone A 66.59 Two diterpene glycosides, virescenosides M 67 and N 68, were isolated from a strain of Acremonium striatisporum associated with the holothurian Eupentacta fraudatrix.60 Mangicols A–G 69–75 are sesterterpene polyols from a Fusarium sp. (strain # CNC-477), tentatively identified as F. heterosporum, that was isolated from driftwood collected in the Bahamas.61 A Fusarium sp. (strain # CNL-619), which was isolated from the green alga Avrainvillea sp. from the US Virgin Islands, produced N-methylsansalvamide 76.62 The structure of sansalvamide A 77, which was isolated from a related Fusarium sp. (strain # CNL-292),63 was confirmed by a rapid, high-yield solid-phase synthesis.64 A Hyphomycetes sp. cultured from a Zooanthus sp. produced the azetinone kasarin 78, which showed weak antibacterial and cytotoxic activities.65 The antimicrobial alkaloids ascosalipyrrolidinones A 79 and B 80 and ascosalipyrone 81 were isolated from an obligate marine fungus Ascochyta salicorniae, which was cultured from Ulva sp., a green alga collected from the German coast of the North Sea.66 An Acremonium sp. isolated from the surface of the tunicate Ecteinascidia turbinata from the Bahamas contained oxepinamides A–C 82–84 and fumiquinazolines H 85 and I 86, of which oxepinamide A 82 showed good anti-inflammatory activity.67 The structures of fumiquinazolines A 87, B 88, F 89 and G 90, which were isolated from a strain of Aspergillus fumigatus from the gastrointestinal tract of the fish Pseudolabrus japonicus,68 have been confirmed by total synthesis.69,70 Penacillazine 91 is a quinoline derivative produced by a Penicillium sp. (strain # 386) from the South China Sea.71
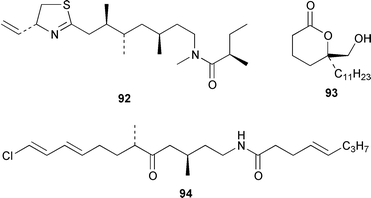
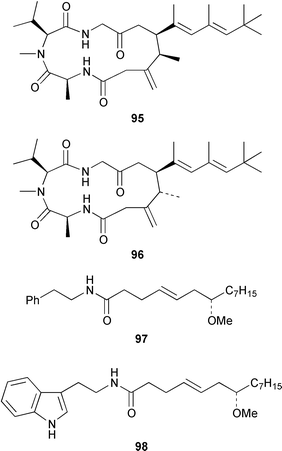
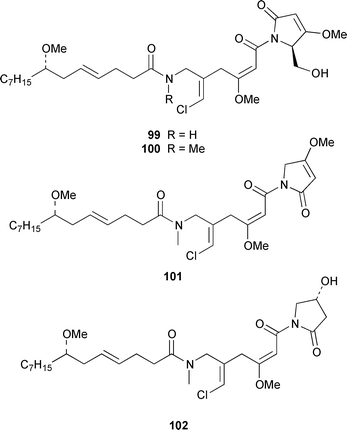
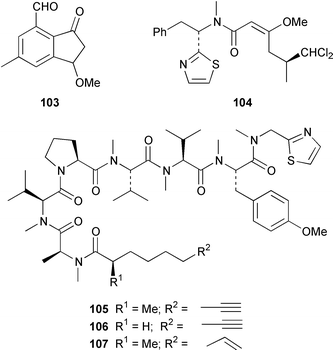
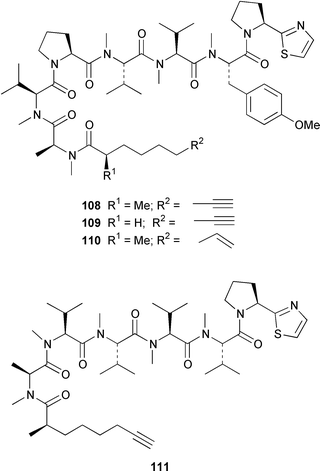
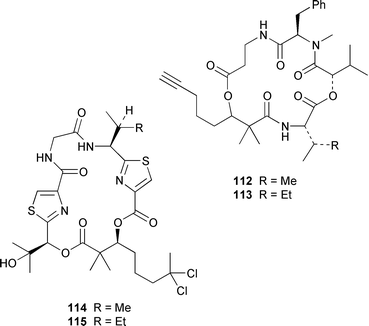
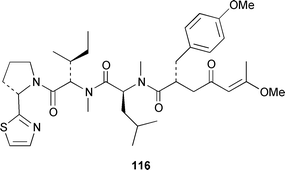
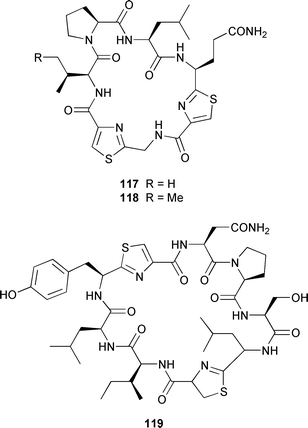
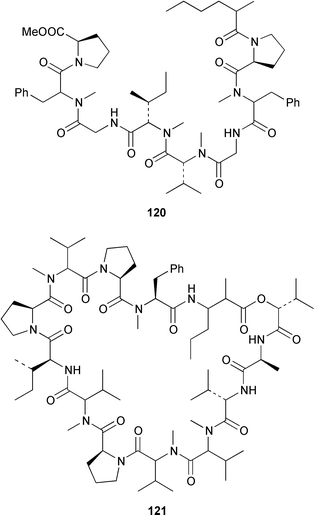
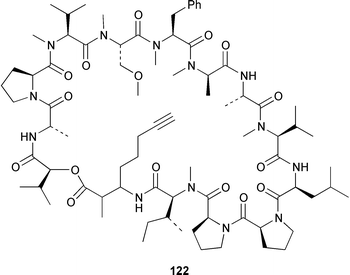
It often appears that Lyngbya majuscula is the only marine cyanobacterium being investigated, which on the basis of its chemical diversity, is not entirely unexpected. Kalkitoxin 92 is an unusual thiazoline derivative from Caribbean specimens of L. majuscula that is ichthyotoxic, cytotoxic, neurotoxic and inhibits IL-1β-induced sPLA2 secretion from HepG2 cells.72 The structure of kalkitoxin 92 was confirmed by total synthesis.72 The structure and absolute stereochemistry of (+)-tanikolide 93, which was isolated from a specimen of L. majuscula from Madagascar,73 have been confirmed by total synthesis.74 The absolute stereochemistry of pitiamide A 94, which was isolated from a mixed assemblage of L. majuscula and Microcoleus sp.,75 was determined by optical rotation studies and confirmed by total synthesis.76 The absolute stereochemistry of antillatoxin, which is a metabolite of L. majuscula from Curaçao,77 was revised from 95 to 96 as the result of synthetic studies.78 A specimen of L. majuscula from Papua New Guinea contained hermitamides A 97 and 98, which are toxic to brine shrimp and mildly toxic to goldfish.79 Malyngamides Q 99 and R 100 are additional members of the series that were isolated from L. majuscula from Madagascar.80 A Hawaiian specimen of L. majuscula contained isomalyngamides A 101 and B 102, which are geometrical isomers of malyngamides A and B about the chloromethylene moiety.81 Malyngamides O and P were isolated from a sea hare. A simple indanone 103 from a specimen of L. majuscula collected in Guam inhibited hypoxia-induced activation of the VEGF gene promotor in Hep3B human liver tumour cells in vitro.82 The structure of dechlorobarbamide 104, from a Curaçao collection of L. majuscula, was elucidated as part of a paper that described the biosynthetic pathway and origin of the chlorinated methyl group in barbamide.83 Two collections of L. majuscula from Apra Harbor, Guam contained a series of linear lipopeptides, apramides A–G 105–111.84 A mixed assemblage of L. majuscula and Schizothrix sp. from Fiji contained the cyclic depsipeptides yanucamides A 112 and B 113, which contain a 2,2-dimethyl-3-hydroxyoct-7-ynoic acid moiety found previously in metabolites of the mollusc Philinopsis speciosa.85 Lyngbyabellins A 114 and B 115 are cytotoxic and antifungal cyclic depsipeptides from specimens of L. majuscula from Guam and the Dry Tortugas National Park, Florida, that contain a 7,7-dichloro-2,2-dimethyl-3-hydroxyoctanoic acid moiety.86–88 The Guam specimen of L. majuscula also contained lyngbyapeptin A 116, previously reported from a Papua New Guinea specimen of L. bouillonii,89 for which the absolute configuration was also determined.87 A Palauan collection of L. majuscula contained dolastatin 3 117, previously obtained as a very minor cytotoxic metabolite from the sea hare Dolabella auricularia,90,91 homodolastatin 3 118 and kororamide 119: dolastatin 3 117 was found to be an inhibitor of HIV-1 integrase.92 Three depsipeptides, malevamides A–C 120–122, were isolated from a specimen of Symploca laeteviridis from Oahu.93
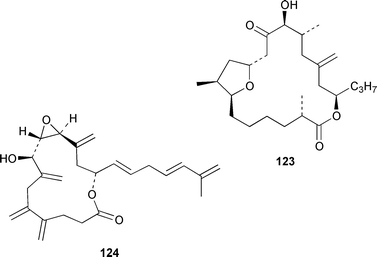
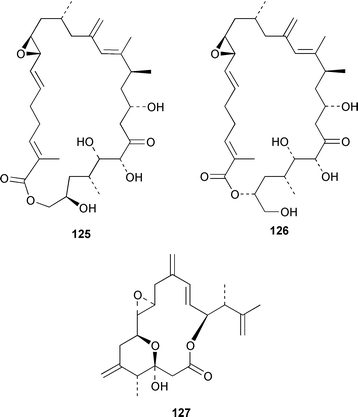
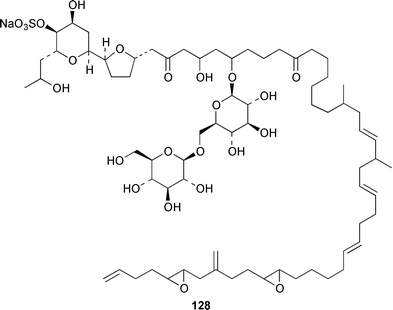
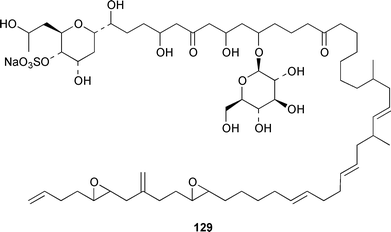
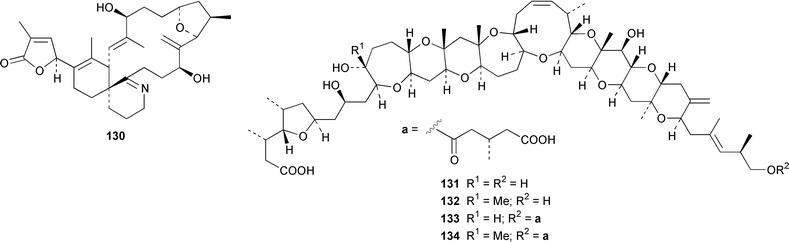
The dinoflagellate Amphidinium sp. (strain # Y-56), which is a symbiont of the flatworm Amphiscolops sp., contained the 19-membered macrolide amphidinolide T 123,94 while Amphidinium sp. (strain # Y-5) produced a 14-membered macrolide, amphidinolide V 124.95 The absolute stereochemistries of amphidinolides G 125 and H 126, which are potent cytotoxic 27- and 26-membered macrolides from Amphidinium sp. (strain # Y-72),96 were determined by X-ray diffraction analysis and interconversion.97 The structure and absolute stereochemistry of (−)-amphidinolide P 127, which is a 15-membered lactone from Amphidinium sp.,98,99 have been confirmed by a convergent total synthesis.100 The structures elucidated for colopsinols D 128 and E 129, which are additional polyhydroxylated metabolites from an Amphidinium sp. (strain # Y-5), lack key stereochemical data.101 Cultures of the dinoflagellate Gymnodinium selliforme, implicated in occurrences of neurotoxic shellfish poisoning in New Zealand, contained gymnodimine B 130.102 A number of ciguatoxin congeners have been proposed on the basis of FAB tandem mass spectrometry.103 The absolute configurations of gambieric acids A–D 131–134, which are potent antifungal polyethers from Gambierdiscus toxicus,104 have been determined by using the modified Mosher method, NMR analysis and chiral HPLC.105
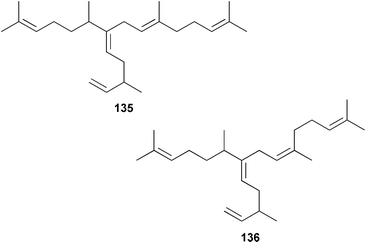
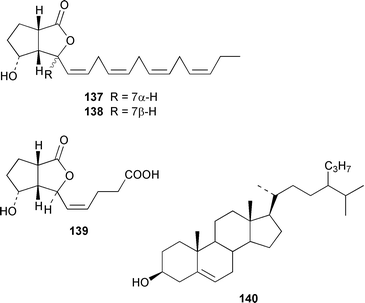
Two important sedimentary sesterterpenoids, the geometrical isomers 135 and 136 have been isolated from the marine diatom Pleurosigma intermedium.106 Bacillariolides I–III 137–139, which are oxylipins from the diatom Pseudonitzschia multiseries,107 have been synthesized from (R)-malic acid.108 The marine chromophyte Aureoumbra lagunensis, known as ‘Texas brown tide’, contains 24-propylcholesterol 140, the structure of which was confirmed by synthesis.109
3 Green algae
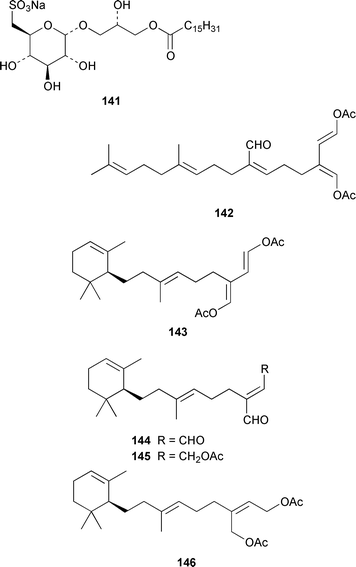
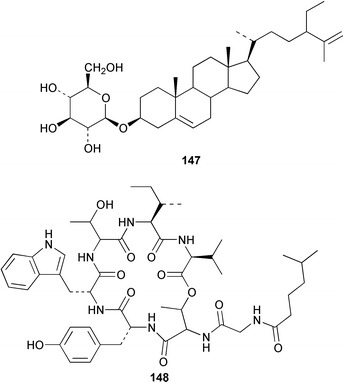
The metabolite of Bryopsis sp. from Oahu that caused ecdysis in the dinoflagellate Gambierdiscus toxicus was identified as 1-O-palmitoyl-3-O-(6′-sulfo-α-D-quinovopyranosyl)-sn-glycerol 141.110 A specimen of Udotea petiolata from the Greek coast contained udoteal B 142, together with several known compounds.111 Four closely-related monocyclic diterpenes 143–146 were isolated from Caulerpa trifaria from Tasmania.112 The antiinflammatory agent produced by Ulva lactuca was identified as 3-O-β-D-glucopyranosylstigmasta-5,25-diene 147.113 An additional depsipeptide, kahalalide O 148, was found in both the green alga Bryopsis sp. from Oahu and the sacoglossan mollusc Elysia ornata that feeds upon it.1144 Brown algae
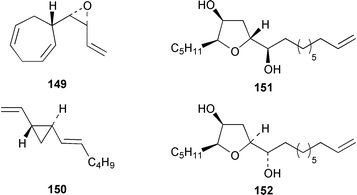
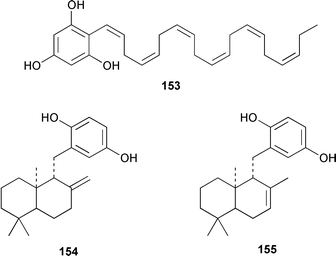
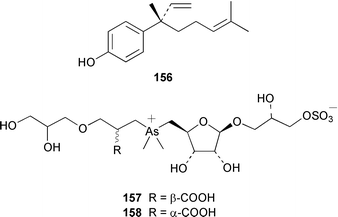
The majority of papers reviewed below concern the synthesis of brown algal metabolites rather than the discovery of new examples. The total synthesis of all four stereoisomers of lamiroxene, which is a spermatozoid-releasing and -attracting pheromone of Laminaria spp.,115 showed that (1′S,2R,3S)-lamiroxene 149 was the most active isomer.116 A total synthesis of (3R,4S)-dictyopterene A 150, which is a sex pheromone of a Hawaiian Dictyopteris sp.,117 used an optically active tributylstannylcyclopropane as a key intermediate.118 (2S,3S,5R)-5-[(1R)-1-Hydroxydec-9-enyl]-2-pentyltetrahydrofuran-3-ol 151 and (2S,3S,5S)-5-[(1S)-1-hydroxydec-9-enyl]-2-pentyltetrahydrofuran-3-ol 152, both of which were isolated from Notheia anomala,119 have been synthesized in an enantiocontrolled manner.120The brown alga Stypopodium schimperi from the Aegean Sea contained the new meroterpenoid schimperiol 153.121 The sesquiterpene hydroquinones zonarol 154 and isozonarol 155 and the corresponding quinones zonarone and isozonarone, which are metabolites of Dictyopteris zonaroides,122 have been synthesized by a common route from β-ionone.123 The structure of sporochnol A 156, which is a fish feeding inhibitor from the Caribbean alga Sporochnus bolleanus,124 has been confirmed by total synthesis of the racemate.125 Two diastereoisomers 157 and 158 of an arsenomethionine-based structure were isolated from Sargassum lacerifolium.126
5 Red algae
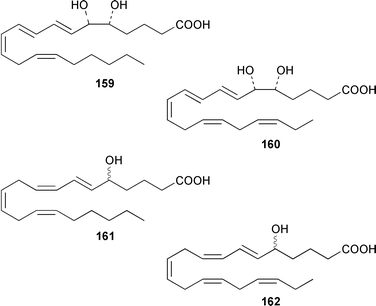
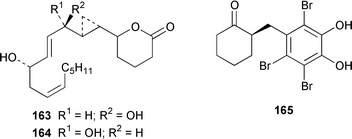
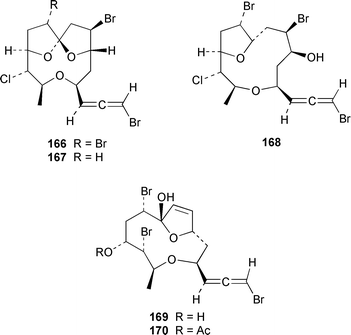
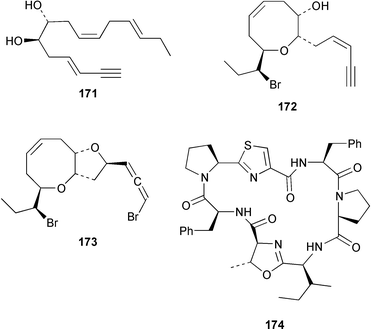
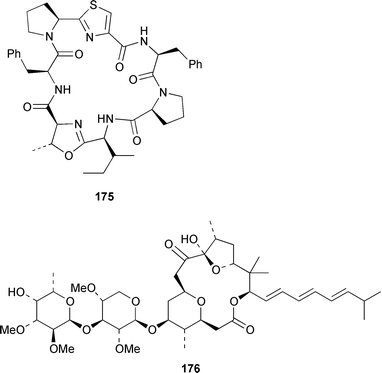
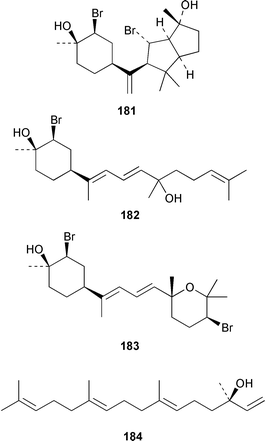
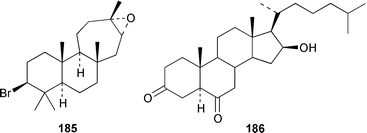
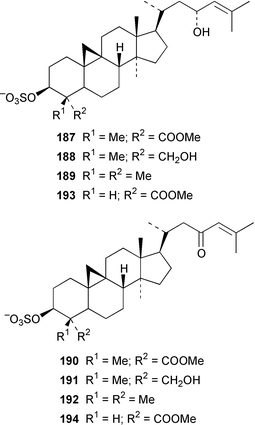
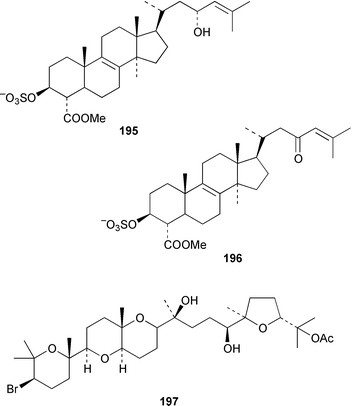
Four oxylipins (5R,6S,7E,9E,11Z,14Z)-5,6-dihydroxyicosa-7,9,11,14-tetraenoic acid 159, (5R*,6S*,7E,9E,11Z,14Z,17Z)-5,6-dihydroxyicosa-7,9,11,14,17-pentaenoic acid 160, (6E,8Z,11Z,14Z)-5-hydroxyicosa-6,8,11,14-tetraenoic acid 161, and (6E,8Z,11Z,14Z,17Z)-5-hydroxyicosa-6,8,11,14,17-pentaenoic acid 162 were isolated from Rhodymenia pertusa.127 The structures of constanolactones A 163 and B 164, which are eicosanoids from Constantinea simplex,128 have been confirmed by total synthesis.129 An unusual fatty ester, pentyl hentriacontanoate, was obtained from an Indian specimen of Chondria armata.130 Several brominated anisoles and cresols were detected by GC-MS from an extract of Polysiphonia sphaerocarpa obtained by a rather harsh steam distillation–solvent extraction technique.131Symphyocladia latiuscula from Korea contained (2R)-2-(2,3,6-tribromo-4,5-dihydroxybenzyl)cyclohexanone 165, which was shown to be a radical scavenger.132 Obtusallenes V 166, VI 167, VII 168, VIII 169 and IX 170, some of which show conformational mobility, were obtained from a Turkish Mediterranean Sea specimen of Laurencia obtusa.133 A highly convergent synthesis of trans-(+)-laurediol 171, which was obtained from Laurencia nipponica,134 was described.135 The syntheses of (+)-prelaureatin 172 and (+)-laurallene 173, both of which are metabolites of L. nipponica,136,137 employed a ring-closing metathesis reaction.138 An Indonesian specimen of the red alga Ceratodictyon spongiosum and its symbiotic sponge Sigmadocia symbiotica contained the antiinflammatory cyclic heptapeptides cis,cis-ceratospongamide 174 and trans,trans-ceratospongamide 175.139 (−)-Polycavernosamide A 176, which is a toxic constituent of Polycavernosa tsudai,140 has been synthesized in a stereocontrolled manner.141Two additional polyhalogenated monoterpenes 177 and 178 were isolated from an Eastern Tasmanian sample of Plocamium cartilagineum.142 The antitumor agent halomon 179 from Portieria hornemannii143 has been synthesized in its racemic form by an efficient 3 step synthesis.144 A palladium-catalyzed cycloreduction of a 1,6-enyne was employed in the synthesis of (±)-laurene 180,145 which is a metabolite of L. glandulifera.146Laurencia microcladia from Il Rogiolo, Tuscany, contained the diterpenes neorogioldiol 181, rogioldiol D 182 and O11,15-cyclo-14-bromo-14,15-dihydrorogiol-3,11-diol 183, together with their putative biosynthetic precursor (−)-geranyllinalool 184.147 The structure and absolute configuration of 3-bromobarekoxide 185, which is an unusual diterpene from a Japanese specimen of L. luzonensis, were determined by X-ray crystallography.148Jania rubens from Tunisia contained a cytotoxic steroid, 16β-hydroxy-5α-cholestane-3,6-dione 186.149 Six cycloartenol sulfates 187–192, two 29-nor-cycloartenol sulfates 193 and 194 and two 29-nor-lanosterol sulfates 195 and 196 were obtained from Tricleocarpa fragilis from Hawaii.150 The protein phosphatase 2A inhibitor, thyrsiferyl 23-acetate 197, which was isolated from Laurencia obtusa,151 has been synthesized by using a convergent strategy.152
6 Sponges
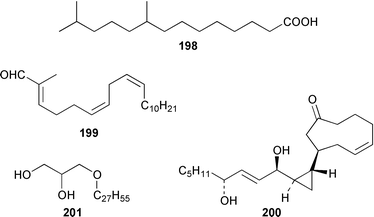
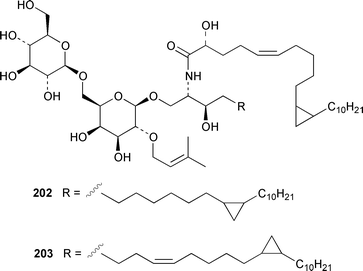
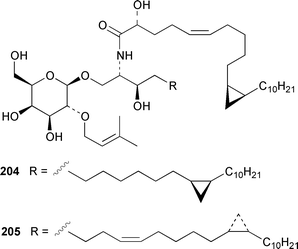
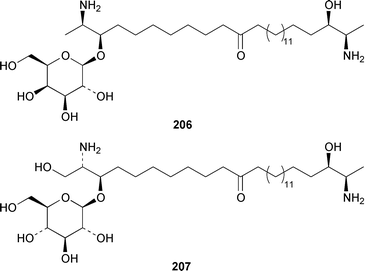
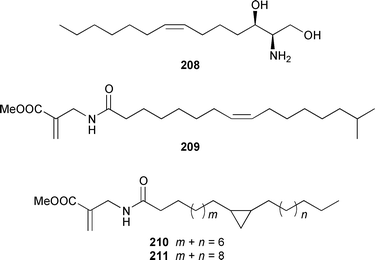
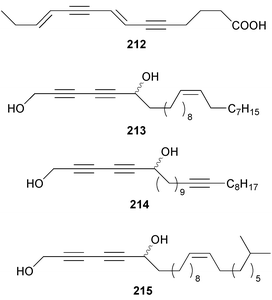
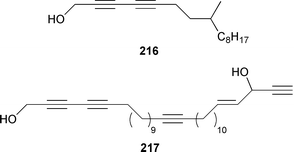
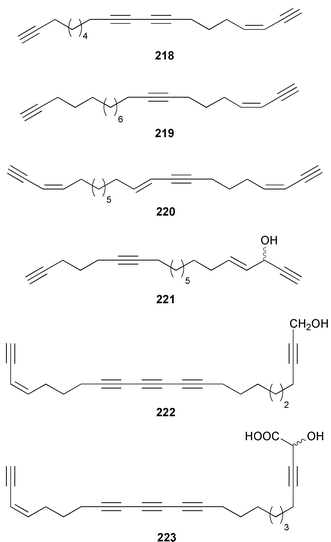
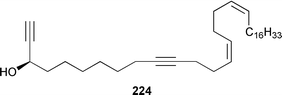
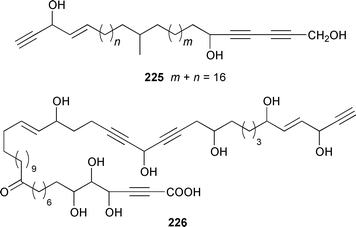
Once again sponges have provided more marine natural products than any other phylum, due in part to their propensity to produce bioactive metabolites. The dimethylated fatty acid 9,13-dimethyltetradecanoic acid 198 was isolated from the Caribbean sponge Calyx podatypa and its structure was confirmed by total synthesis.153 The calcareous sponge Leucetta microraphis contained (2E,6Z,9Z)-2-methylicosa-2,6,9-trienal 199, which exhibited moderate cytotoxicity.154 Halicholactone 200, which is a lipoxygenase inhibitor from Halichondria okadai,155 was synthesized by using the chirality of (diene)Fe(CO)3 complexes to induce asymmetry.156 A South China Sea specimen of Pachychalina sp. contained 3-heptacosoxypropane-1,2-diol 201.157 Two additional prenylated glycosphingolipids, plakosides C 202 and D 203, were isolated from Ectyoplasia ferox from the Bahamas.158 The structures of plakosides A 204 and B 205, which were reported as immunosuppressive agents from Plakortis simplex,159 have been confirmed by total synthesis but the synthetic materials were less active than previously reported.160 The absolute configurations of (−)-rhizochalin 206, which is a metabolite of the calcareous sponge Rhizochalina incrustata from Madagascar,161 and oceanapiside 207, which is an antifungal agent from Oceanapia phillipensis,162 were determined by using circular dichroism.163,164Haliclona vansoesti from Curaçao contained large quantities (5% dry wt.) of (2R,3R,7Z)-2-aminotetradec-7-ene-1,3-diol 208.165 Three additional N-acyl-2-methylene-β-alanine methyl esters, hurghamides E–G 209–211, were isolated from a Red Sea species of Hippospongia.166The antimicrobial constituent of a Japanese Oceanapia sp. was identified as the bis-acetylene 212.167 Strongylodiols A 213, B 214 and C 215, which occur as enantiomeric mixtures of different ratios (A: 91% R, B: 97% R, C: 84% R), are cytotoxic acetylenic alcohols from a Strongylophora sp. from Okinawa.168 A Taiwanese specimen of S. durissima contained two additional acetylenes, durissimols A 216 and B 217, as well as a new meroditerpenoid.169 Six polyacetylenes, aikupikanynes A–F 218–223 were obtained from a Callyspongia sp. from the Red Sea.170 An Indonesian Haliclona sp. contained lembehyne A 224, which induced neuritogenesis in pheochromocytoma PC12 and neuroblastoma Neuro 2A cells.171 An additional polyacetylene, pellynol I 225 was isolated from a Pellina sp. from Tonga.172 The polyacetylene carboxylic acid haliclonyne 226 was obtained from a Haliclona sp. from the Red Sea.173
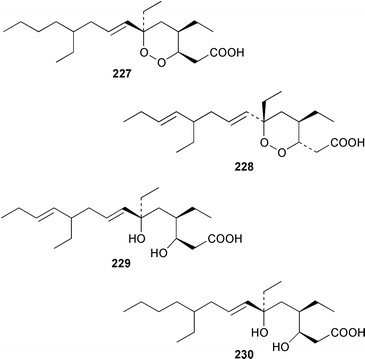
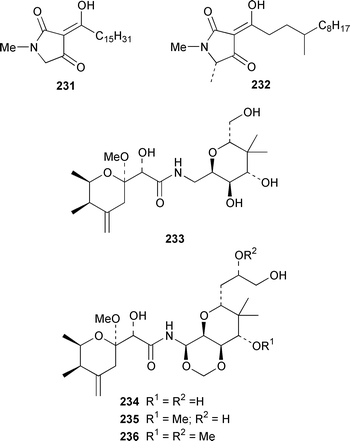
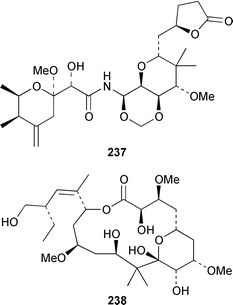
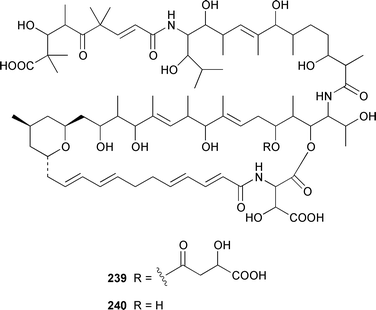
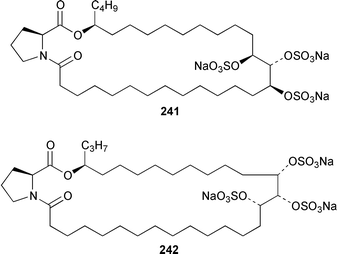
A Bahamian specimen of Plakortis simplex contained two additional cytotoxic cyclic peroxides, plakortides I 227 and J 228, together with seco-plakortides H 229 and I 230.174Melophlus sarassinorum from Indonesia contained melophlins A 231 and B 232, which reverse the phenotype of ras-transformed cells.175 Mycalamides C 233 and D 234 are additional cytotoxins from a newly described Australian Stylinos sp. from Heron Island.176 Mycalamide D 234 was also isolated from a Mycale sp. from New Zealand.177 Mycalamides A 235 and B 236, which were obtained from a New Zealand Mycale sp.,178 and theopederin D 237 from a Theonella sp.,179 have been synthesized.180,181 A New Zealand Mycale sp. also contained peloruside A 238, which is a potent cytotoxic macrolide.182 The macrolide lactams, chondropsins A 239 and B 240, are tumor cell growth inhibitors from a Chondropsis sp. from Wollongong, Australia.183 A specimen of Penares sp. from Japan contained penarolide sulfates A1241 and A2242, which were shown to be α-glucosidase inhibitors.184
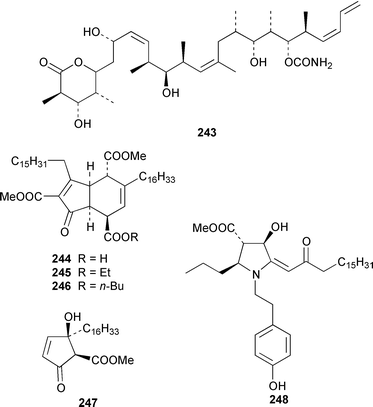
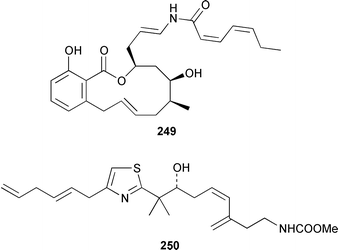
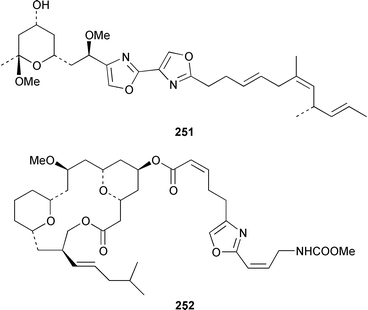
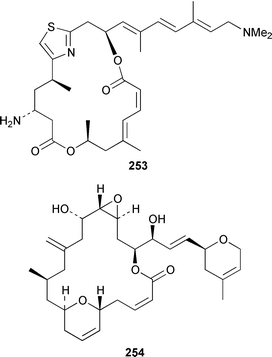
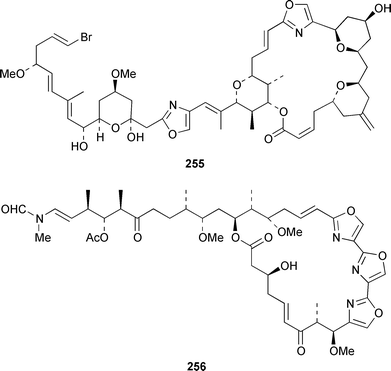
The total synthesis of sponge metabolites, particularly macrolides, appears to be a very popular research area. In addition to a detailed description of the evolution of a gram-scale synthesis of discodermolide 243,185 which is an anti-cancer agent from Discodermia dissoluta,186 a total synthesis featuring boron-mediated aldol reactions has been reported.187,188 Manzamenones A 244, C 245 and F 246 and the proposed biosynthetic precursor untenone A 247, all of which are metabolites of a Plakortis sp.,189,190 have been synthesized as the corresponding racemates from 2-furanacetonitrile.191 The structure of another metabolite of a Plakortis sp.,192 (2S,3S,4R)-plakoridine A 248, has been confirmed by total synthesis.193 The absolute configuration of salicylihalamide A 249, which is a cytotoxin from a Haliclona sp.,194 was revised as a result of an asymmetric total synthesis.195,196 The first total synthesis of (−)-mycothiazole 250,197 which was isolated from Spongia mycofijiensis, employed a convergent strategy: the optical rotation of the synthetic product was significantly larger, albeit of the same sign, than that of the natural material.198 An additional synthesis of (−)-hennoxazole A 251, which is an antiviral agent from a Polyfibrospongia sp.,199 has been accomplished by using a convergent approach.200 The structure and absolute configuration of leucascandrolide A 252, which is an antifungal and cytotoxic metabolite of Leucascandra caveolata,201 has been confirmed by total synthesis.202 A total synthesis of (−)-pateamine 253, which is an immunosuppressive agent from Mycale sp.,203 employed a concise and convergent route.204 (−)-Laulimalide 254 from a Hyattella sp.,205 also known as fijianolide B from Spongia mycofijiensis,206 has been synthesized in a stereocontrolled manner.207 The total synthesis of phorboxazole B 255, which is an antifungal and cytotoxic macrolide from Phorbas sp.,208 has been accomplished in a stereoselective manner.209,210 The structure and stereochemistry of (−)-mycalolide A 256, which is an antifungal and cytotoxic trisoxazole from Mycale sp.,211 have been confirmed by total synthesis.212,213
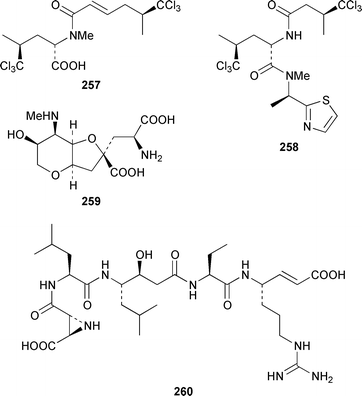
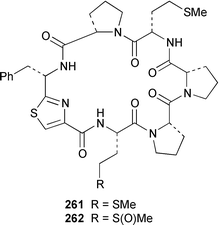
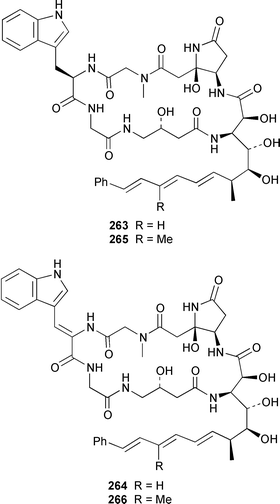
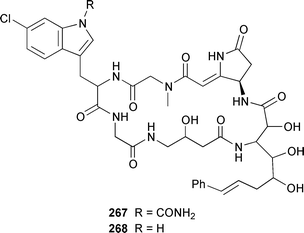
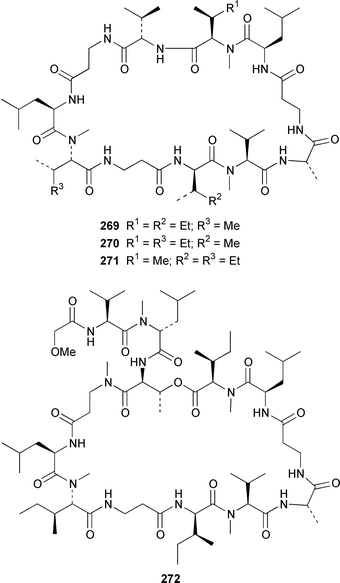
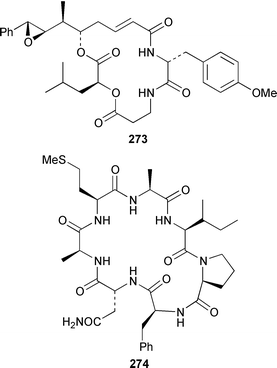
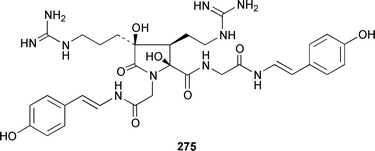
Herbacic acid 257, which is the simplest to date of the 5,5,5-trichlorinated leucine derivatives, was isolated from a Great Barrier Reef specimen of Dysidea herbacea.214 A specimen of D. herbacea from the Great Barrier Reef contained (−)-neodysidenin 258, the absolute configuration of which was determined by capillary electrophoresis of Marfey's derivatives.215 The structure and stereochemistry of (−)-dysiherbaine 259, which is a neurotoxic amino acid from D. herbacea,216 have been confirmed by three independent total syntheses.217–219 Miraziridine A 260, which was isolated from a Japanese lithistid sponge Theonella aff. mirabilis, is a most unusual linear pentapeptide that inhibited the protease cathepsin B.220 A specimen of Haliclona nigra from Papua New Guinea contained two cyclic hexapeptides, haligramides A 261 and B 262, that showed an unusual pattern of selectivity in the National Cancer Institute's 60 cell-line panel.221 Microsclerodermins F–I 263–266 are additional cytotoxic and antifungal cyclic peptides that were isolated from a deep-water Microscleroderma sp. from Palau.222Theonella cupola from Okinawa contained dehydromicroscerodermins C 267 and D 268, the stereochemistries of which are not defined.223 The cyclic peptides barangamides B–D 269–271 and the cyclic depsipeptide theonellapeptolide IIe 272 are additional metabolites of a specimen of T. swinhoei from Indonesia.224 An additional synthesis of arenostatin A 273, which is a cytotoxin from Dysidea arenaris,225 provides a route that is amenable to structural modifications in the side chain.226 Both solution-phase and solid-phase syntheses of phakellistatin 5 274, which was reported to be a cytotoxic constituent of Phakellia costada,227 have been accomplished but the products, while chemically identical, were not biologically identical.228 (±)-Anchinopeptolide D 275, which is a metabolite of Anchinoe tenacior,229 has been synthesized in seven steps in 10% yield.230
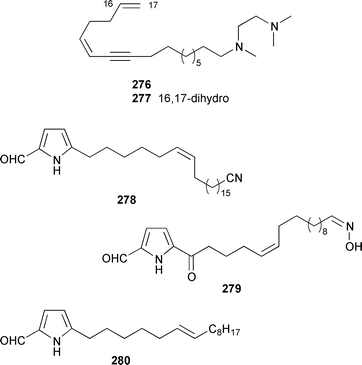
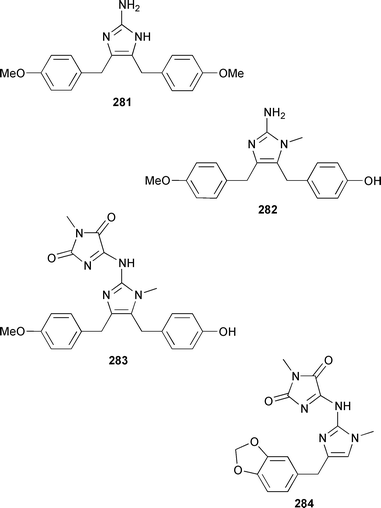
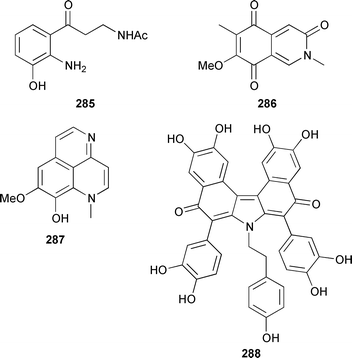
Two unusual acetylenic alkaloids, clathculins A 276 and B 277, were isolated as an inseparable mixture from a South African specimen of Clathrina aff. reticulum.231Mycale tenuispiculata from the Thiruvananthapuram coast of India contained three additional 5-alkylpyrrole-2-carbaldehyde derivatives, (6′Z)-5-(23′-cyanotricos-6′-enyl)pyrrole-2-carbaldehyde 278, mycaleoxime 279 and (6′E)-5-(pentadec-6′-enyl)pyrrole-2-carbaldehyde 280.232 The calcareous sponge Leucetta cf. chagosensis from the Egyptian Red Sea contained an additional imidazole alkaloid, naamine D 281, that was both antifungal and inhibited nitric oxide synthase.233 The structures of naamine A 282 and naamidine A 283, which are metabolites of L. chagosensis,234 have been confirmed by total synthesis.235 The structure of clathridine A 284, which was obtained from Clathrina clathrus,236 has been confirmed by total synthesis.237 Erebusinone 285, a simple pigment from the Antarctic sponge Isodictya erinacea, caused a significant reduction of molting and increased mortality when fed to sympatric predatory amphipods.238 Mimosamysin 286, which was identified as a constituent of Reniera,239Petrosia240 and Xestospongia spp.,241 has been synthesized in eight steps with a 13% overall yield.242 Isoaaptamine 287, which is a protein kinase C inhibitor from Aaptos aaptos,243,244 has been synthesized together with several analogues.245 The structure of purpurone 288, which is an alkaloid from an Iotrochota sp.,246 has been confirmed by total synthesis.247
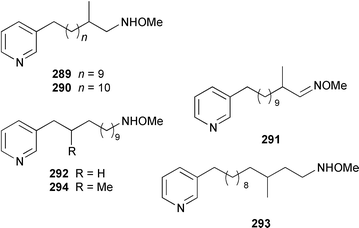
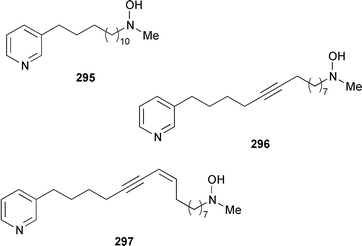
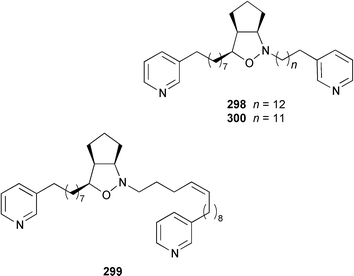
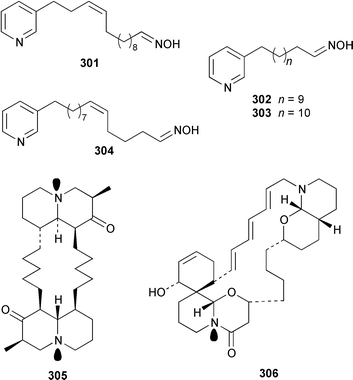
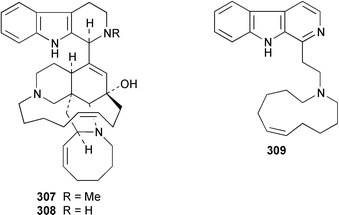
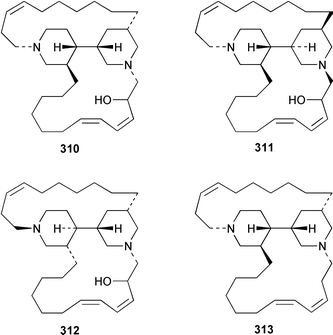
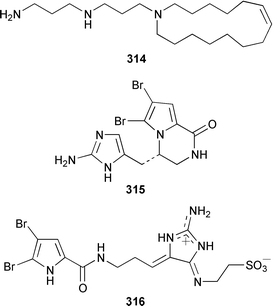
Cribochalines A 289, a moderately active antifungal agent, and B 290 are 3-alkylpyridine alkaloids from a specimen of Cribochalina sp. from Pohnpei that also contained ikimine A 291248 as a 2.8 ∶ 1 mixture of the (S)- and (R)-enantiomers.249 The moderately cytotoxic 3-alkylpyridine alkaloids hachijodines A 292, B 293, C 289 and D 294 were isolated from a Japanese Xestospongia sp. while hachijodines E–G 295–297 were isolated from an Amphimedon sp. from Japan.250 The structures of cribochaline A 289 and hachijodine C are identical but the reported spectral data, albeit in different solvents, are strikingly different. Pyrinodemins B–D 298–300 are additional potent cytotoxic bis-pyridine alkaloids that were isolated from an Okinawan Amphimedon sp. together with the antimicrobial 3-alkylpyridine alkaloids 301–304.251 A specimen of Xestospongia exigua from Papua New Guinea contained xestosin A 305, the structure of which was determined by X-ray crystallography.252 An Indonesian Echinochalina sp. contained a very unusual macrocyclic alkaloid, 'upenamide 306, the complete stereochemistry of which, although drawn, remains to be determined.253N-Methyl-epi-manzamine D 307, the structure of which was determined by X-ray analysis, and epi-manzamine D 308 were isolated as cytotoxic agents from an unidentified Palauan sponge.254 A synthesis of manzamine C 309, which is an alkaloid from a Haliclona sp.,255 involved the use of the Ramburg–Bäcklund rearrangement for the formation of the aza-macrocycle.256 The endemic Brazilian sponge Arenosclera brasiliensis contained arenosclerins A–C 310–312 and haliclonacyclamine E 313.257 Motuporamine C 314, which is a metabolite of Xestospongia exigua from Papua New Guinea,258 has been synthesized by using a ring-closing metathesis reaction.259
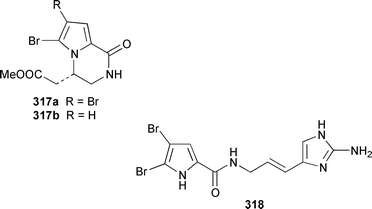
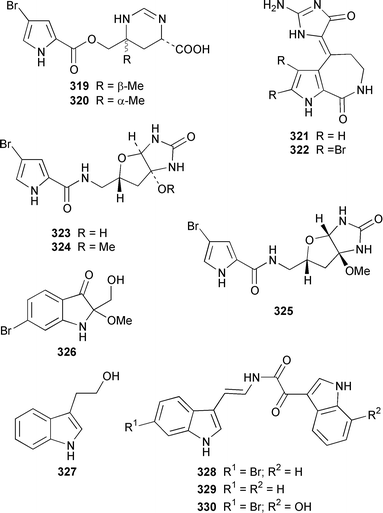
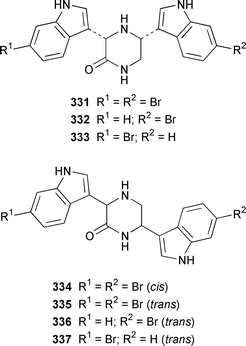
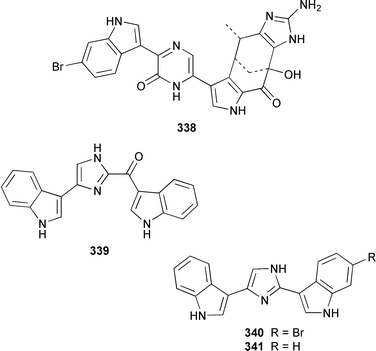
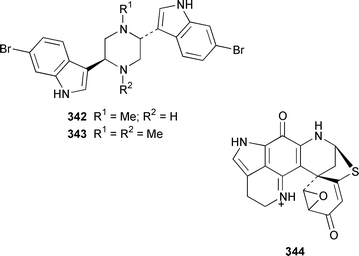
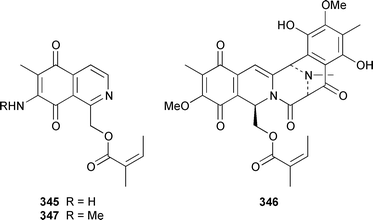
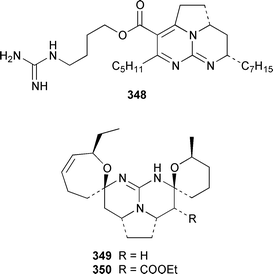
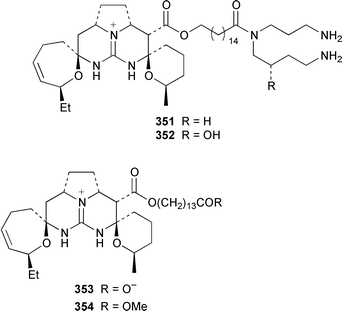
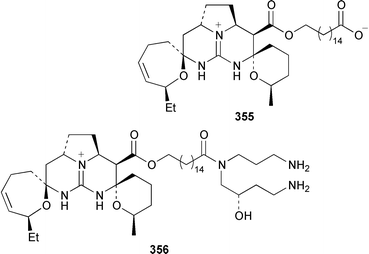
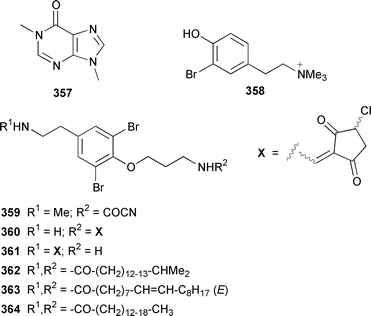
Two additional members of the oroidin family of alkaloids, cyclooroidin 315 and the antihistaminic agent taurodispacamide A 316, were isolated from Agelas oroides from the Bay of Naples.260Agelas ceylonica from the Mandapam coast of India yielded (S)-(+)-hanashin methyl ester 317a,261 rather than the racemate reported previously from a Homaxinella sp. from Japan.262 A late-arriving paper reported the isolation of 3-debromohanishin methyl ester 317b from an Indian specimen of Axinella tenuidigitata.592 Both oroidin 318, which is a metabolite of Agelas oroides,263 and the corresponding (Z)-isomer have been synthesized.264 The absolute configurations of manzacidins A 319 and C 320, which were isolated from an Okinawan Hymeniacidon sp.,265 have been determined by total synthesis.266 (Z)-Debromohymenialdisine 321, which was first isolated from Phakellia flabellata,267 has been synthesized in four steps and 34% overall yield: (Z)-3-bromohymenialdisine 322, which was isolated from Stylissa carteri,268 was an intermediate in the synthesis.269 The total syntheses of slagenins A 323, B 324 and C 325, recently isolated from Agelas nakamurai,270 have also been reported.271 Matemone 326 is a mildly cytotoxic and antimicrobial bromo-indole derivative from Iotrochota purpurea from Matebo Island in the western Indian Ocean.272 A specimen of Ircinia spinulosa from Turkey contained the plant auxin tryptophol 327.273 A Coscinoderma sp. from Papua New Guinea contained three bis-indole alkaloids, coscinamides A–C 328–330.274Rhaphisia lacazei from the Mediterranean contained seven additional bis-indole alkaloids 331–337 of the dihydrohamacanthin family.275 Dragmacidin F 338 is an antiviral bromoindole alkaloid from a Halicortex sp. from the Italian coast.276 Topsentin A 339, which was first obtained from Topsentia genitrix,277 and nortopsentins B 340 and D, 341, which are metabolites of Spongosorites ruetzleri278 and Dragmacidon sp.,279 respectively, have been synthesized in a concise manner.280 Both dragmacidins A 342 and B 343, which were isolated from a Hexadella sp.,281 have been synthesized in good yields.282,283 Two Antarctic sponges of the family Latrunculidae, Latrunculia sp. and Negombata sp., contained the antibacterial agent discorhabdin R 344.284 A Cribrochalina sp. from the Maldives contained three isoquinoline alkaloids, cribrostatins 3–5 345–347, as minor antimicrobial agents.285 The polycyclic guanidine alkaloid dehydrobatzelladine C 348 was isolated from Monanchora arbuscula from Belize while crambescidins 359 349 and 431 350 were obtained from M. unguiculata from the Seychelles.286 The syntheses of ptilomycalin A 351, which was isolated from a Red Sea Hemimycale sp.287 and a Caribbean Batzella sp.,288 originally identified as Ptilocaulis aff. spiculifera,287 crambescidins 800 352 and 657 353 from the related Mediterranean sponge Crambe crambe,289 and neofolitispate 2 354 from an Indian Neofolitispa dianchora,290 confirmed the earlier stereochemical assignments and established the (S)-absolute stereochemistry of the hydroxyspermidine residue in crambiscidin 800 352.291 A similar synthetic strategy was used for the total syntheses of 13,14,15-isocrambescidins 657 355 and 800 356, which are metabolites of Crambe crambe,289,292 again confirming the relative and absolute stereochemistries.293 A Spongosorites sp. from Southern Australia contained the base 1,9-dimethylhypoxanthine 357.294
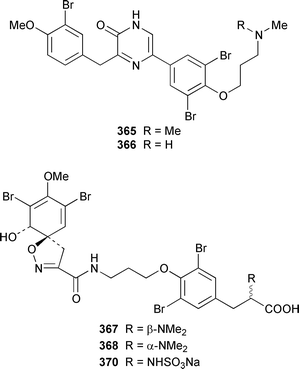
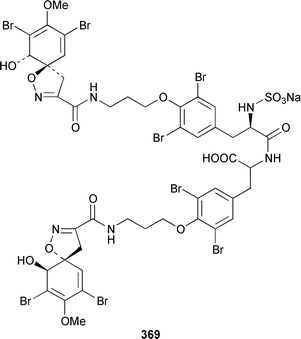
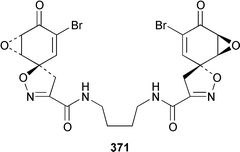
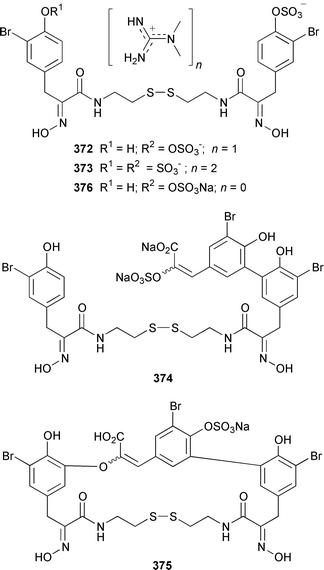
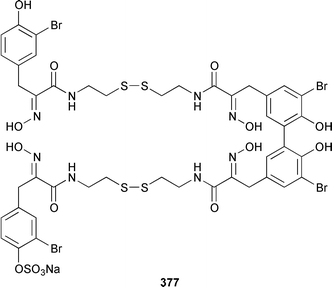
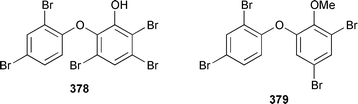
Only one additional minor bromotyrosine derivative 358 was isolated from Verongula gigantea from the Bahamas.295 An undescribed Verongid sponge from Molokai contained N-methylceratinamine 359 and wai'anaeamines A 360 and B 361.296 The mololipids 362–364, which inhibit HIV-1, were also isolated from a Hawaiian Verongid sponge as an inseparable mixture of compounds, the structures of which were elucidated by hydrolysis and GC-MS analysis of the lipid components.297 A Suberea sp. from Okinawa contained the cytotoxic pyrazin-2(1H)-ones, ma'edamines A 365 and B 366.298 Ianthesines A–D 367–370, which showed potent Na,K-ATPase activity, are additional dibromotyrosine derivatives from an Australian Ianthella sp.299 A specimen of Aplysina gerardogreeni from the Sea of Cortez contained the interesting bis-epoxide calafianin 371.300 Four additional cytotoxic bromotyrosine metabolites that also inhibit farnesyl protein transferase and leucine aminopeptidase, psammaplins A1372 and A2373, and aplysinellins A 374 and B 375, were isolated from specimens of Aplysinella rhax collected at both Pohnpei and Palau.301 Another specimen of A. rhax from the Great Barrier Reef contained psammaplin A 11′-sulfate 376 and bisaprasin 11′-sulfate 377, both of which inhibited [3H]-1,3-dipropyl-8-cyclopentylxanthine binding to rat-brain adenosine A1 receptors.302 An additional polybrominated diphenyl ether 378, the structure of which was determined by X-ray analysis, was isolated from Dysidea herbacea from the Great Barrier Reef.303 A Dysidea sp. from Lizard Island contained 2-(2′,4′-dibromophenoxy)-4,6-dibromoanisole 379, together with two sesquiterpenes (see below).304
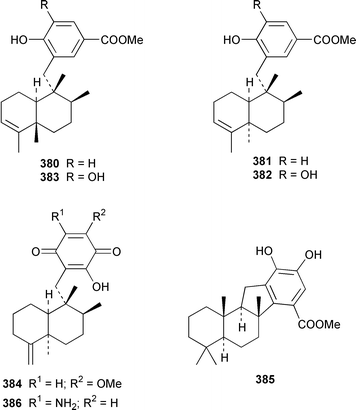
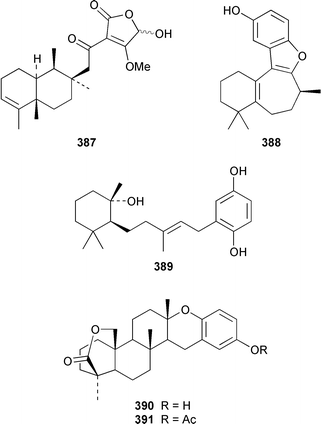
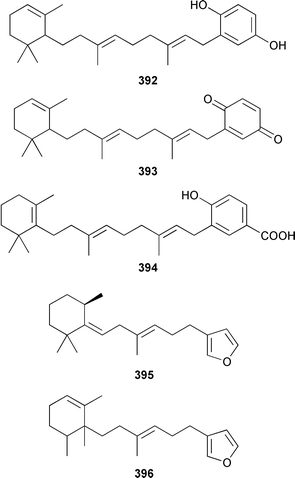
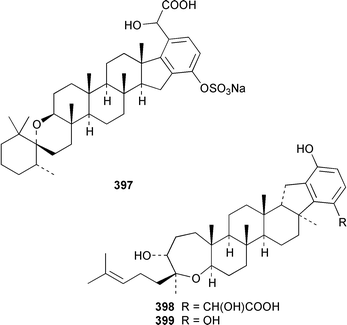
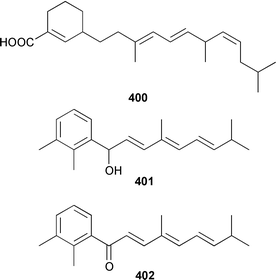
A Hyrtios sp. from the Seychelles contained the merosesquiterpenoids hyrtiophenol 380, 5-epi-hyrtiophenol 381, 18-hydroxy-5-epi-hyrtiophenol 382 and 18-hydroxyhyrtiophenol 383, while H. tubulatus from Curaçao contained 21-hydroxy-19-methoxyarenarone 384 together with known metabolites.305 Pelorol 385 was reported from a Great Barrier Reef specimen of Dactylospongia elegans and, accompanied by 5-epi-smenospongine 386, from Petrosiaspongia metachromia from Yap, Micronesia.306,307 A Dysidea sp. from Vanuatu contained the oxidized and rearranged merosesquiterpene dysidotronic acid 387, which selectively inhibited phospholipase A2.308 The structure of frondosin B 388, which was isolated from Dysidea frondosa,309 was confirmed by total synthesis.310 Fulvanin 2 389, which is a merosesquiterpenoid from Reniera fulva,311 has been synthesized from (−)-sclareol.312 Strongylophorines 9 390 and 11 391 are additional meroditerpenoids from Strongylophora durissima from Taiwan.313,169 The meroditerpenoids cacospongins B–D 392–394 were obtained, together with the diterpene furan cacospongin A 395, from a Cacospongia sp. from the Philippines.314 The structure of cacospongin A was recently revised from 395 to 396.315 Adociasulfate 10 397, which inhibits the kinesin family of motor proteins, is an additional merohexaprenoid sulfate from Haliclona (aka Adocia) sp. from Palau.316 A Haliclona sp. from Indonesia contained two new merotriterpenes, haliclotriols A 398 and B 399.317 A Clathria sp. from southern Australia contained clathrins A–C 400–402, which might be examples of meroterpenoids or rearranged terpenoids.318
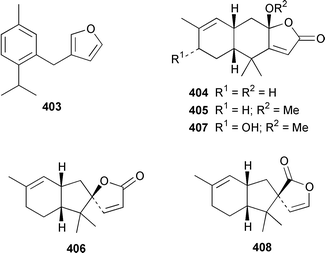
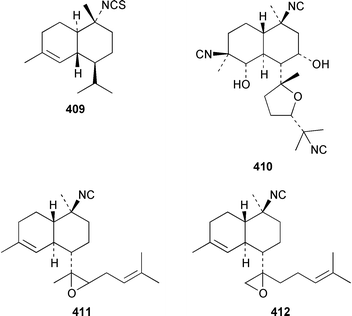
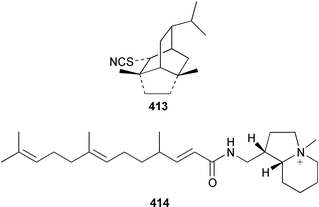
The structure of tsitsikammafuran 403, which was isolated from a Dysidea sp. from South Africa, was confirmed by total synthesis from thymol.319 A specimen of D. fragilis from southern India contained (+)-furodysinin lactone 404, (+)-O-methylfurodysinin lactone 405 and spirofragilide 406.320 A specimen of D. herbacea from the Great Barrier Reef yielded 6-hydroxyfurodysinin-O-methyl lactone 407 and dehydroherbadysidolide 408, in addition to the polybrominated diphenyl ether 379 (see above).304 Great Barrier Reef specimens of Acanthella cavernosa contained 10-isothiocyanatocadin-4-ene 409, 8-hydroxyisokalihinol 410 and two isocyanobifloradiene epoxides 411 and 412.321 Both enantiomers of 2-thiocyanatoneopupukeanane 413, which was isolated from both Phycopsis terpnis and Axinyssa aplysinoides,322,323 have been synthesized from (R)-carvone.324 The absolute configuration of the sesquiterpene alkaloid stellettamide A 414, which is a metabolite of a Stelletta sp.,325 was confirmed by total synthesis of its enantiomer.326
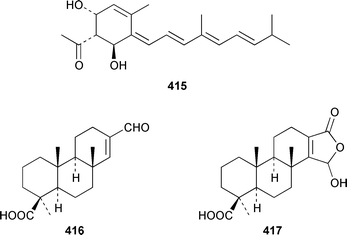
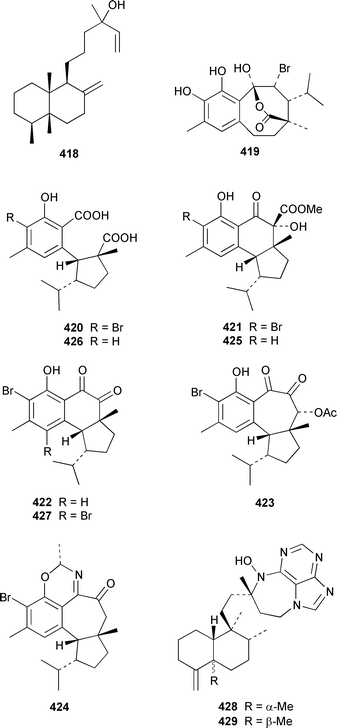
Phorbasin A 415 is an unstable diterpene polyene from a southern Australian Phorbas sp.327 A specimen of Coscinoderma mathewsi from Pohnpei contained the aldehyde 416 and the γ-hydroxybutenolide 417, which appears to be identical to spongiabutenolide A.328,329 The relative configuration at C-13 in nakamurol A 418, which is a metabolite of Agelas nakamurai from Okinawa,330 has been tentatively assigned as the result of total synthesis of the racemate.331 A reinvestigation of the constituents of Hamigera tarangaensis has resulted in the reassignment of the structure of 419332 to hamigeran E 420 and the isolation of hamigerans A–D 421–424, debromohamigerans A 425 and E 426 and 4-bromohamigeran B 427, among which hamigeran B 422 showed in vitro antiviral activity and others were moderately cytotoxic.333 Asmarines A–F 428–433 are cytotoxic diterpene alkaloids that contain an adenine residue from a Raspailia sp. from Eritrea.334 A Hippospongia sp. from Goa, India contained ent-untenospongin A 434.335 The structure of luffarin W 435, which was obtained from Luffariella geometrica,336 was confirmed by a total synthesis that involved the thermal rearrangement of an ozonide.337
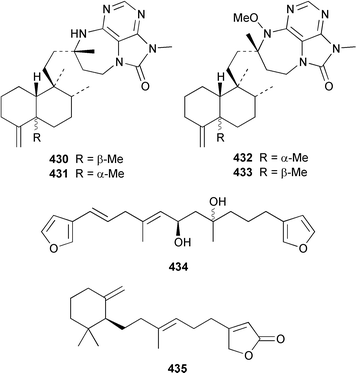
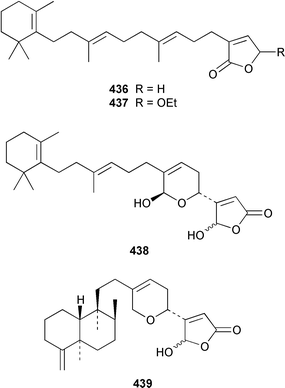
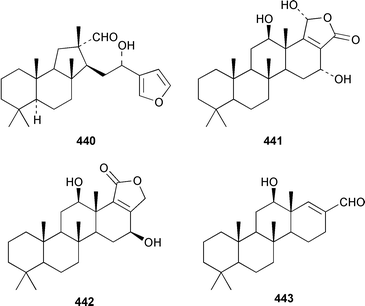
Two additional sesterterpenes 436 and 437 were isolated from Hyrtios cf. erecta from Fiji but the major bioactive compounds of this sponge were known.338 The absolute stereochemistries of the known antiinflammatory agents manoalide 438 and cacospongionolide B 439 were determined by comparison of their CD spectra with those of synthetic model compounds.339 The absolute configuration of (−)-hyrtiosal 440, which is a cytotoxic sesterterpene from H. erectus (= erecta ?),340 was determined by synthesis from sclareol.341 Three additional scalaranes, hyrtiolide 441, 16-hydroxyscalariolide 442 and 12-deacetyl-Δ17-hyrtial 443 were obtained from an Okinawan specimen of H. erectus.342
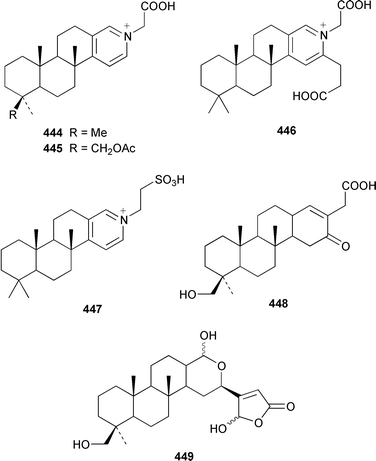
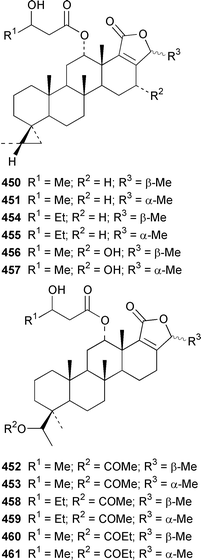
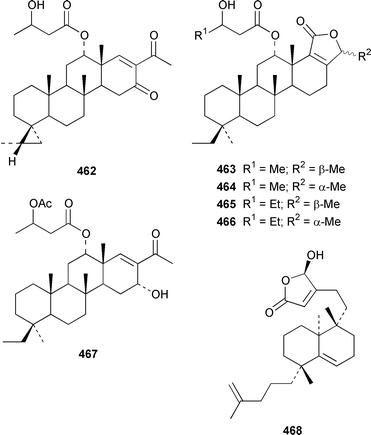
Four sesterterpene pyridinium alkaloids, spongidines A–D 444–447, together with 21-hydroxypetrosiaspongiolides K 448 and P 449, were isolated as antiinflammatory agents from a Spongia sp. from Vanuatu.343Strepsichordaia aliena from Indonesia contained a total of eighteen new bishomoscalaranes, honulactones A–L 450–461, honu'enone 462, phyllofolactones H–K 463–466 and phyllofenone 467: honulactones A–D 450–453, of which the structures of 451 and 453 were determined by X-ray analysis, were shown to be cytotoxic.344,345 Four additional syntheses of the cytotoxic agent dysidiolide 468, which was isolated from Dysidea etheria,346 have been reported.347–350
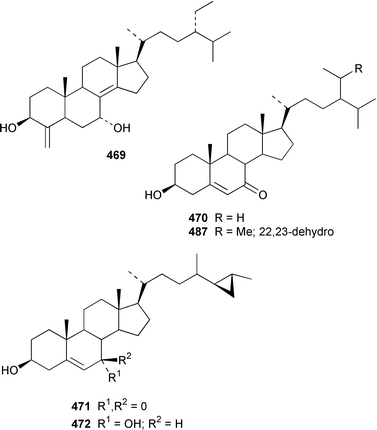
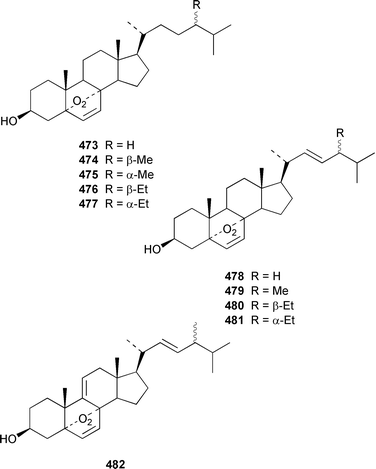
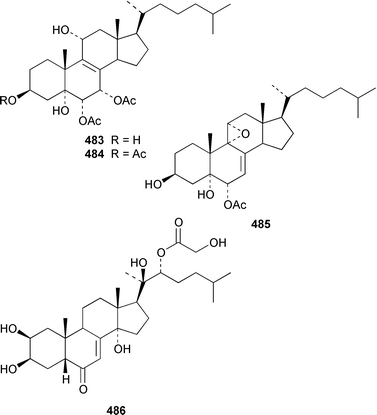
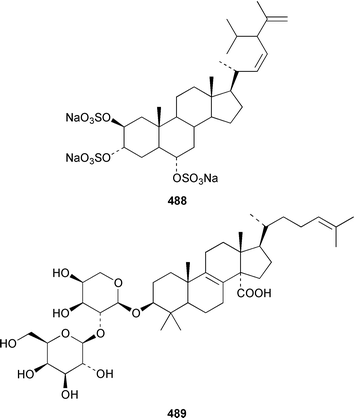
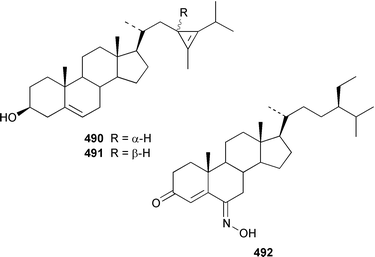
A specimen of Theonella swinhoei from the Philippines contained 7α-hydroxytheonellasterol 469 as a mildly cytotoxic constituent of the sterol fraction.351Polymastia sobustia from the South China Sea contained 3β-hydroxystigmast-5-en-7-one 470.352 Two 26,27-cyclosterols 471 and 472 were isolated from a Japanese specimen of Strongylophora corticata.353 A mixture of 5α,8α-epidioxy sterols 473–482 was identified as a cytotoxic component of Luffariella cf. variabilis.354 A Dysidea sp. from Northern Australia contained three polyoxygenated sterols 483–485 that inhibit the binding of IL-8 to the human recombinant IL-8 receptor type A.355 The ecdysteroid 2β,3β,14α,20β-tetrahydroxy-22α-(2-hydroxyacetyloxy)-5β-cholest-7-en-6-one 486 was obtained from Iotrochota birotulata from the Bahamas.356 Polysterol A 487 and polysterol B sulfate 488 were obtained from a Japanese Epipolasis sp.357 Specimens of Erylus formosus from the Bahamas contained eryloside F 489, a potent thrombin receptor antagonist that inhibits human platelet aggregation in vitro.358 (23R)-23H-Isocalysterol 490, a metabolite of Calyx niceansis,359 and (23S)-23H-isocalysterol 491, which was obtained from C. podotypa,360 have both been synthesized.361 (6E,24R)-24-Ethyl-6-hydroxyiminocholest-4-en-3-one 492, which was isolated from Cinachyrella spp. sponges,362 was synthesized from β-sitosterol in 30% overall yield.363
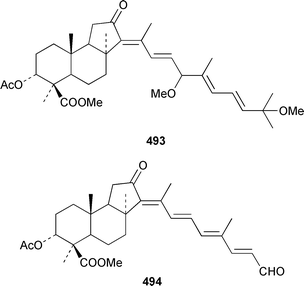
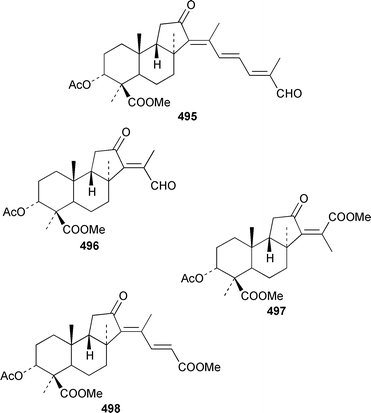
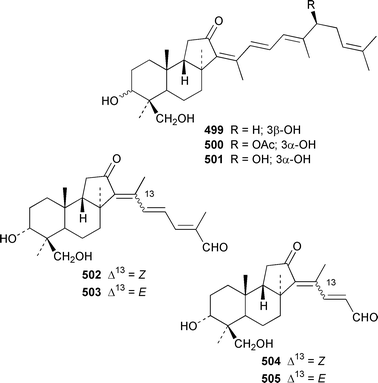
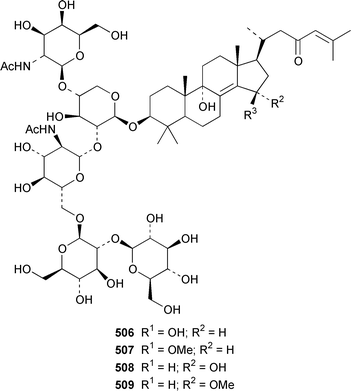
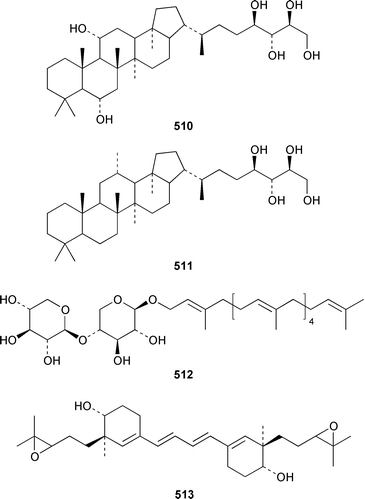
Six additional cytotoxic isomalabaricane derivatives, globstellatic acid E methyl ester 493, 3-O-acetyljaspiferal methyl esters B 494, D 495 and G 496 and the dimethyl esters of jaspiferoic acids A 497 and B 498, were obtained from a new species of Jaspis from Vanuatu, after methylation with diazomethane to obtain the corresponding methyl esters.364 Three additional isomalabaricanes, 29-hydroxystelliferin D 499, 3-epi-29-hydroxystelliferin E 500 and 3-epi-29-hydroxystelliferin A 501, that induce morphological changes in rat fibroblasts, were obtained from a Japanese specimen of Stelletta globostellata.365 Aurorals 1–4 502–505 are truncated isomalabaricanes from Rhabdastrella globostellata from New Caledonia.366Melophlus isis from Guam contained four additional triterpenoid saponins 506–509.367 A Petrosia sp. from Eritrea contained bacteriohopanehexol 510, which is proposed to be a metabolite of an associated bacterium.368 12-Methylbacteriohopanetetraol 511 is the first such 12-methylhopanoid from Plakortis simplex from the Bahamas.369 The same specimen of P. simplex contained the unusual heptaprenylglycoside, plakopolyprenoside 512.370 The proposed structure of naurol A 513, which was isolated from an unidentified Pacific sponge,371 has been disproved by total synthesis.372
7 Coelenterates
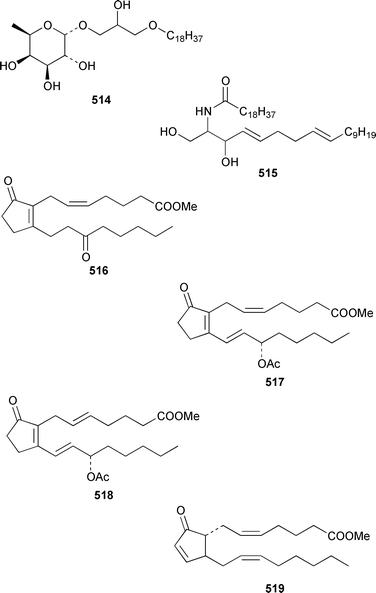
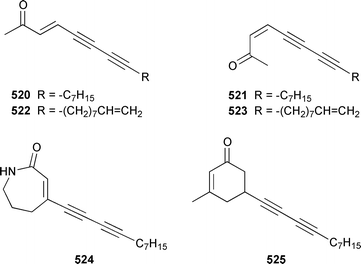
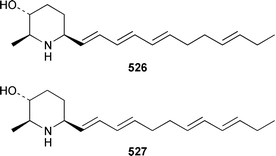
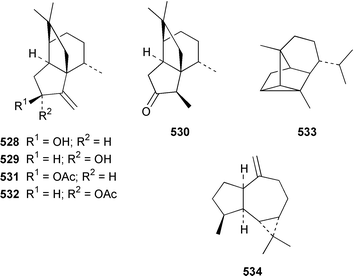
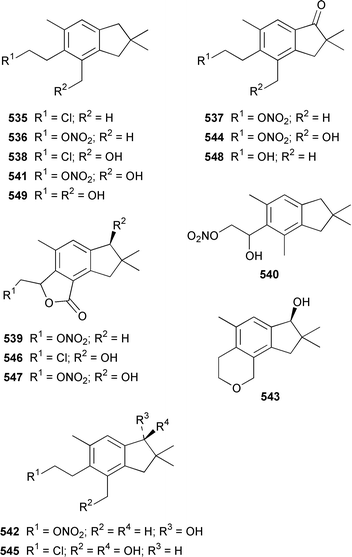
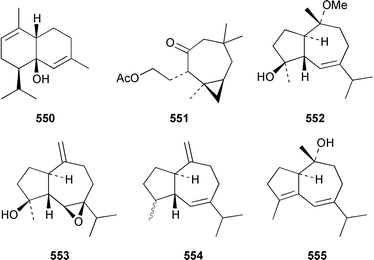
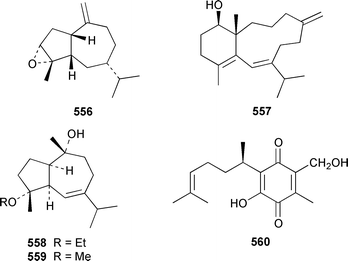
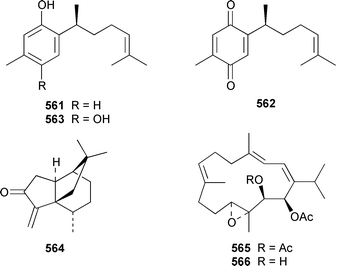
The chemistry of coelenterates (gorgonians, soft corals, hydroids, etc.) was again dominated by reports of diterpenes, sesquiterpenes and polyhydroxylated sterols. Among the few lipids reported was 2-hydroxy-3-(octadecyloxy)propyl-α-D-fucopyranoside 514, which was isolated from both a Sinularia sp. and S. gravis from India.373 Humesamide 515 is a ceramide from the soft coral Cladiella humesi from Hainan, China.374 An unusual 15-ketoprostaglandin 516 was isolated from Sarcophyton crassocaule from the Indian Ocean.375 Three additional prostaglandins, (5Z,15S)-15-acetylprostaglandin B2 methyl ester 517, (5E,15S)-15-acetylprostaglandin B2 methyl ester 518 and (5Z,13Z)-9-oxoprosta-5,10,13-trienoic acid methyl ester 519, were obtained as minor metabolites of Plexaura nina from the Bahamas.376 The scleractinian coral Montipora sp. from Korean waters contained six acetylenic compounds, montiporynes A–F 520–525.377 The hydroid Corydendrium parasiticum contained two isomeric piperidinol alkaloids, corydendramines A 526 and B 527, which were isolated as potent fish feeding inhibitors.378Five suberosane sesquiterpenes, suberosenol A 528, suberosenol B 529, suberosanone 530, suberosenol A acetate 531 and suberosenol B acetate 532, were isolated as cytotoxic components of the gorgonian Isis hippuris.379 Studies of the volatile oils of the soft corals Clavularia viridis and Sarcophyton acutangulum from Japan resulted in the isolation of cyclosinularane 533 and (+)-alloaromadendrene 534, the enantiomer of the known terrestrial plant metabolite, respectively.380 Alcyopterosins A–O 535–549 are sesquiterpenes of the illudin class that were isolated from Alcyonium paessleri from South Georgia Island: the nitrate esters 536, 537, 539–542, 544 and 547 are the first to have been encountered as natural products.381 6-Hydroxy-α-muurolene 550 was isolated as an antifungal constituent of a Heteroxenia sp. from the Philippines.382 An additional seco-africanane, 4-acetoxy-3,15-dinor-2,3-seco-african-2-one 551, was isolated from an Indian specimen of Sinularia dissecta.383 The Indian soft coral Nephthea chabrollii contained four additional sesquiterpenes, 10α-methoxy-4β-hydroxyguai-6-ene 552, 6β,7β-epoxy-4α-hydroxyguai-10-ene 553, 4,15-dihydroguaia-6,10-diene 554 and guaia-4,6-dien-10α-ol 555.384 An Indian Nephthea sp. contained (1S,3R,4S,5S,7S)-3,4-epoxyguaia-10(12)-ene 556, together with a new diterpene 557 and known compounds.385Sarcophyton buitendijki from the Andoman and Nicobar Islands contained 10α-hydroxy-4α-methoxyguai-6-ene 558 and 4α-ethoxy-10α-hydroxyguai-6-ene 559, together with known compounds.386 The Caribbean gorgonian Pseudopterogorgia rigida contained mochiquinone 560 along with known aromatic sesquiterpenes.387 (+)-Curcuphenol 561, (−)-curcuquinone 562 and (+)-curcuhydroquinone 563, which are antimicrobial metabolites of Pseudopterogorgia rigida,388 have been synthesised by using Brewer's yeast to accomplish the resolution step.389 Suberosenone 564, which is a metabolite of Subergorgia suberosa,390 has been synthesized as the corresponding racemate.391
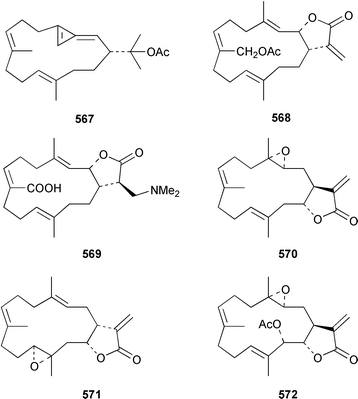
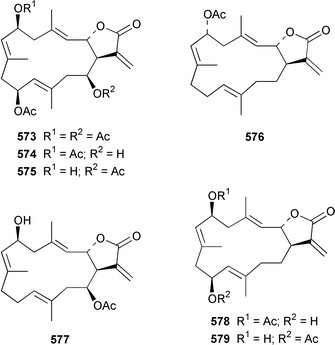
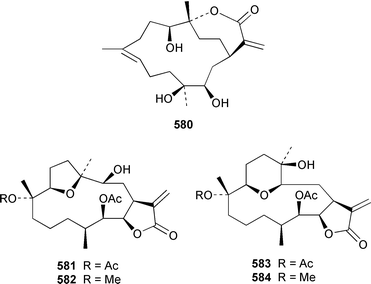
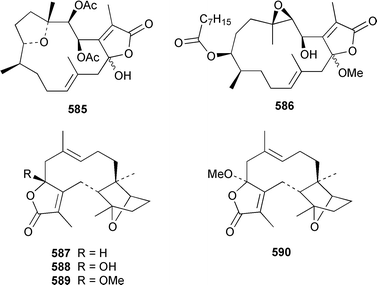
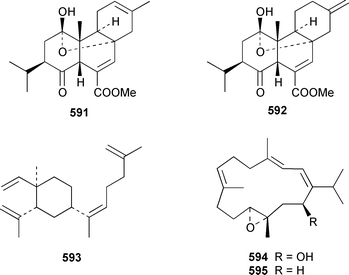
The soft coral Cladiella kashmani from Mozambique contained flaccidoxide 13-acetate 565 together with the known compound flaccidoxide 566,392 the absolute stereochemistry of which was determined for the first time.393 A specimen of Nephthea brassica from Taiwan yielded an unusual cembranoid 567 that incorporated a methylenecyclopropene moiety.394 Lobocrassolide 568 is a cytotoxic cembranoid from a Taiwanese specimen of Lobophytum crassum.395 A Lobophytum sp. from the Philippines contained the cembranoid 569, formally derived by addition of dimethylamine to lobohedleolide, as an HIV-inhibitory agent.396 Three cytotoxic cembranoids, sarcocrassolide 570, crassolide 571 and 13-acetoxysarcocrassolide 572, were isolated from Sarcophyton crassocaule from Taiwan.397 Seven cembranoids 573–579, one of which 573 exhibited significant cytotoxicity, were obtained from Clavularia koellikeri from Okinawa.398 Capillolide 580 was isolated together with known cembranoids from Sinularia capillosa from Hainan Island in the South China Sea.399,400 As part of a paper describing the synthesis of analogues of uprolides D–G, which are cembranoids from Eunicea mammosa,401,402 the structures of uprolide F diacetate and uprolide G acetate were revised from 581 and 582 to 583 and 584, respectively.403Pachyclavularia violacea from Papua New Guinea contained two cembranoids, pachyclavulariolides E 585 and F 586, and four simple briaranes, pachyclavulariolides A–D 587–590.404 The structure of pachyclavulariolide B 588 was confirmed by X-ray crystallography and pachyclavulariolide F 586 was cytotoxic.404 A Sarcophyton sp. from Kalipur Island, India, contained two additional tricyclic diterpenes, sarcophytin B 591, the structure of which was determined by X-ray crystallography and sarcophytin C 592.405Sinularia inelegans from Taiwan contained the cytotoxic lobane hydrocarbon 593.406
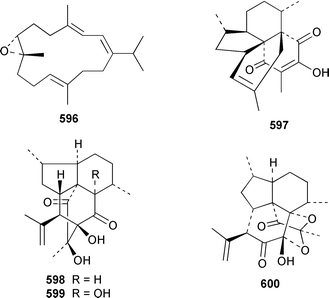
A number of cembranoids from soft corals have been synthesized. The absolute stereochemistry of (+)-11,12-epoxysarcophytol A 594, which was isolated from an Australian Lobophyton sp.,407 has been determined by total synthesis.408 (+)-11,12-Epoxy-11,12-dihydrocembrene C [(+)-11,12-epoxycembrene C] 595 from Sinularia grayi,409 and (−)-7,8-epoxy-7,8-dihydrocembrene C 596 from Sarcophyton crassocaule,410 have been synthesized by using enantioselective routes.411,412
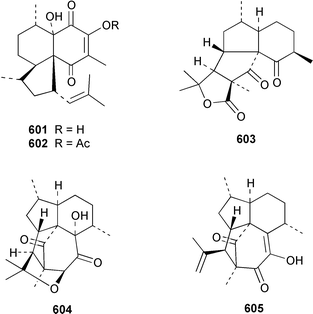
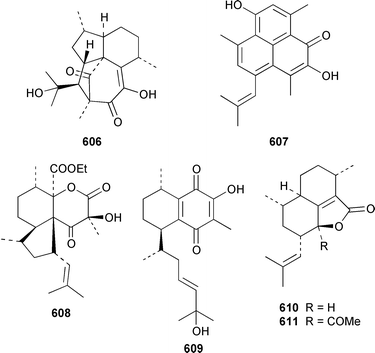
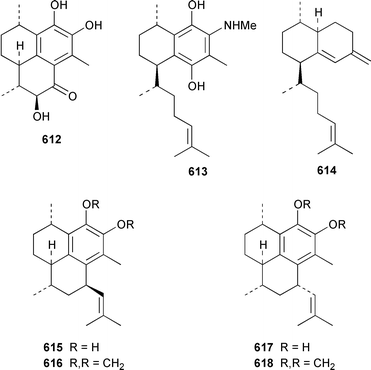
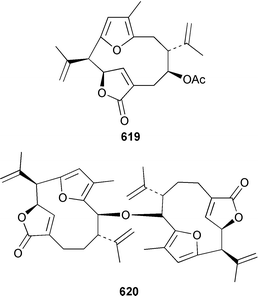
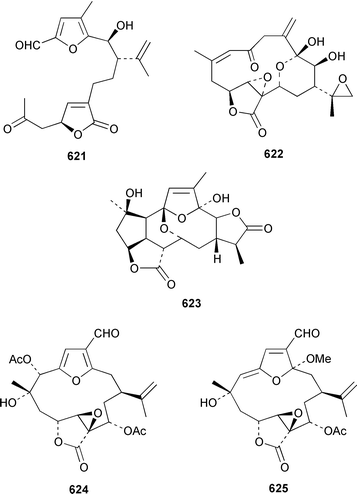
There have been a relatively large number of studies of the Caribbean gorgonian Pseudopterogorgia elisabethae, resulting in the discovery of some very interesting diterpenes. A specimen of P. elisabethae from Columbia contained columbiasin A 597, which possessed the new colombiane carbon skeleton.413 Cumbiasins A–C 598–600 from the same specimen of P. elisabethae incorporate two more new carbon skeletons called cumbiane and seco-cumbiane, respectively.414 The same specimen later yielded elisabethin D 601, elisabethin D acetate 602, 3-epi-elisabanolide 603, elisapterosins A 604, B 605, which displayed strong in vitro anti-tuberculosis activity, and elisapterosins C 606, elisabatin C 607, elisabetholide 608, the benzoquinone 609, amphilectolide 610, 4-acetylamphilectolide 611 and amphiphenalone 612.415–417 The structures of elisabethin D 601, 3-epi-elisabanolide 603 and elisapterosin B 605 were determined by using X-ray crystallography.415 Elisabethamine 613 is a diterpene alkaloid from P. elisabethae from the Florida Keys.418 Elisabethatriene 614 was identified as a key intermediate in the biosynthesis of the pseudopterosins in P. elisabethae.419 The structures of the aglycone of pseudopterosins G–J from P. elisabethae420 and helioporin E from Heliopora coerulea421 were revised from 615 and 616 to 617 and 618 respectively as the result of a total synthesis.422 12-Acetoxypseudopterolide 619 from P. elisabethae from the Florida Keys showed modest anti-cancer activity.423 A specimen of P. bipinnata from Columbia contained the dimeric pseudopterane, biskallolide A 620, which appears to be a solvolysis product of kallolide.424 The same specimen of P. bipinnata also contained seco-bipinnatin J 621, bipinnatolide K 622 and verrillin 623.425,426 Among the fish feeding inhibitors isolated from the Brazilian gorgonian Lophogorgia violacea were two additional lophotoxin derivatives, 7-acetoxy-8-hydroxylophotoxin 624 and 3-methoxy-8-hydroxylophotoxin 625.427
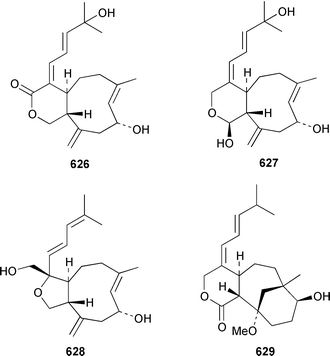
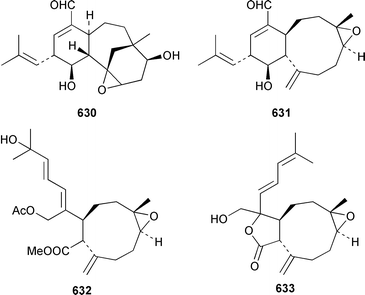
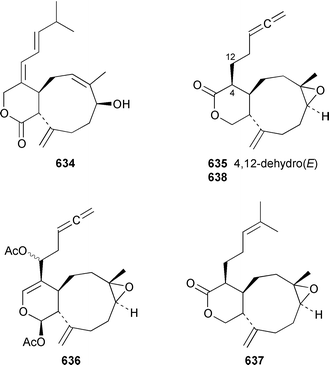
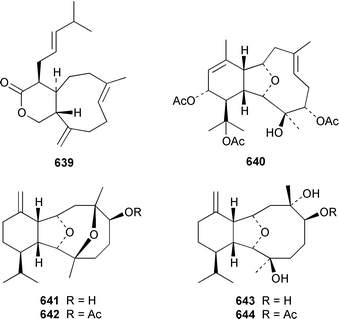
As part of a paper that reported the absolute configurations of xeniolide-A 626 and xenialactol 627, a new xenicane-type diterpene, xeniaoxolane 628, was identified in a Xenia sp. from Okinawa.428 Six additional xenicanes, 11-O-methylflorlide A 629, 2β-epoxyfloridicin 630, xeniafaraunol B 631 and florlides F–H 632–634, were obtained from a Japanese specimen of X. florida.429Acalycigorgia inermis from Korea contained four additional xenicanes, acalycixeniolides D–G 635–638: the structure assigned as acalycixeniolide C in this paper has been reassigned as acalycixeniolide G.430 The structure of coraxeniolide-A 639, which is a metabolite of the pink coral Corallium sp.,431 has been confirmed by total synthesis.432
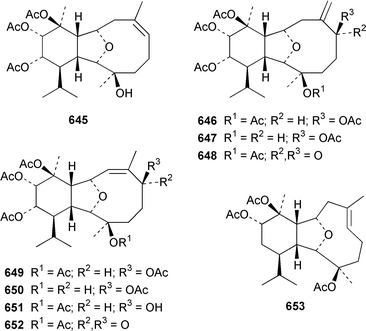
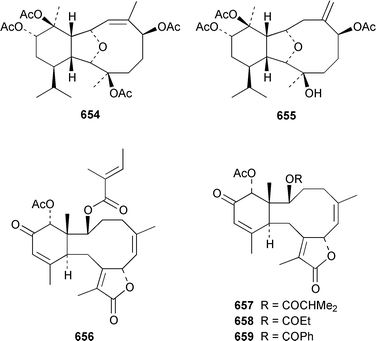
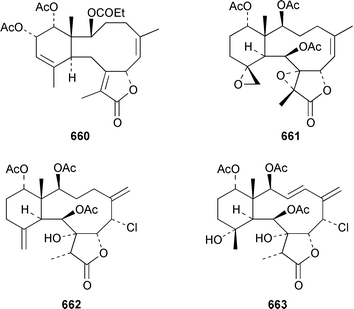
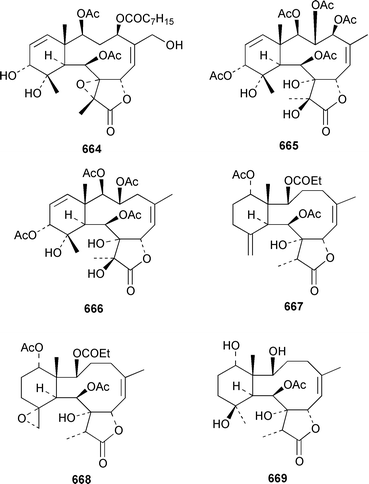
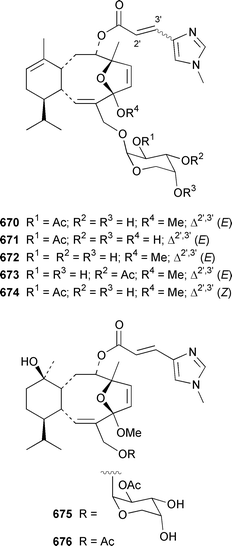
The gorgonian Muricella sp. from Korea contained muricellin 640, which is a diterpenoid of the cladiellin class that is mildly cytotoxic and inhibits phospholipase A2.433 The structures of sclerophytins A and B, which were originally isolated from Sclerophytum capitalis from Eniwetak,434 have been revised from 641 and 642 to 643 and 644, respectively.435 Two total syntheses of the proposed structure 641 of (−)-sclerophytin A clearly disproved the proposed structure.436,437Eunicella cavolinii from Marseille contained eight additional cladiellins 645–652, while E. singularis from the same location contained three related metabolites 653–655.438 Anthoptilides A–E 656–660 are relatively simple members of the briarane class from the Australian sea pen Anthoptilum cf. kukenthali: anthoptilides B 657 and C 658 inhibited the binding of [3H]-1,3-dipropyl-8-cyclopentylxanthine on adenosine A1 receptors.439 Umbraculolides B–D 661–663 are additional briaranes from the Indian gorgonian Gorgonella umbraculum.440 A Japanese Briareum sp. has yielded three additional briaranes, violides N–P 664–666, of which violide N 664 exhibited moderate cytotoxicity.441 Junceellolides E–G 667–669 are additional briaranes from the gorgonian Junceella fragilis from Taiwan.442 The gorgonian Erythropodium caribaeorum from several sites in the Southern Caribbean contained the known microtubule stabilizing compound eleutherobin 670, which had previously been isolated from a rare Eleutherobia sp. from Western Australia,443 together with desmethyleleutherobin 671, desacetyleleutherobin 672, isoeleutherobin 673, Z-eleutherobin 674, caribaeoside 675 and caribaeolin 676.444
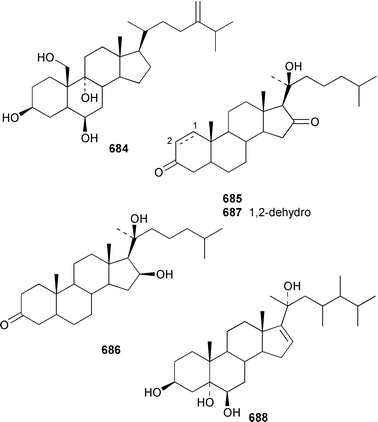
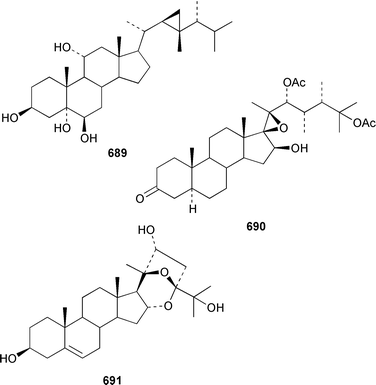
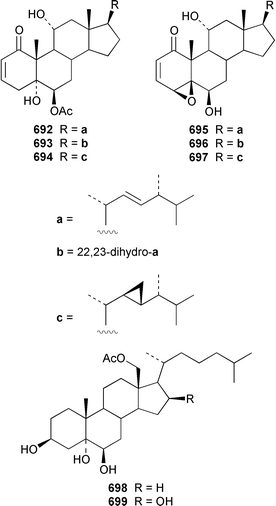
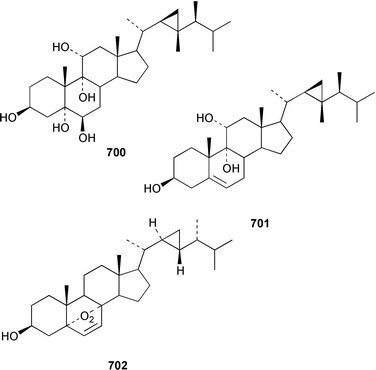
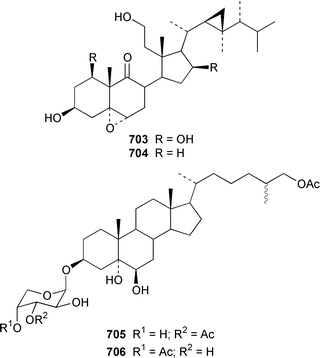
Two 17β,20β-epoxysterols 677 and 678 and a dihydroxygorgost-5-ene 679 were obtained from the Indian Ocean soft coral Sarcophyton crassocaule.445 The gorgonian Acalycigorgia inermis from Korea contained three cytotoxic sterols 680–682.446 Melithasterol A 683, which is a metabolite of the gorgonian Melithaea ocracea,447 has been synthesized from the corresponding sterol peroxide by microwave irradiation.448 The soft coral Sinularia inexplicata from the South China Sea yielded 24-methylenecholesta-3β,6β,9α,19-tetraol 684.352 Three cytotoxic steroids, (20S)-20-hydroxycholestane-3,16-dione 685, (16S,20S)-16,20-dihydroxycholestan-3-one 686 and (20S)-20-hydroxycholest-1-ene-3,16-dione 687, were obtained from the Spanish gorgonian Leptogorgia sarmentosa.449 The soft coral Sarcophyton trocheliophorum from Singapore contained a cytotoxic steroid 688, the side-chain stereochemistry of which remains to be determined.450 Sarcoaldosterol A 689 is an antifungal agent from a Heteroxenia sp. from the Philippines.382 The structure of hippuristerone A 690, which was obtained from the Taiwanese gorgonian Isis hippuris, was determined by X-ray crystallography.451 The polyhydroxylated sterol 691 from an Indian Ocean specimen of Gorgonella umbraculum contained an unusual side-chain ketal functionality.452 Six 1-ketosterols, yonasterols A–F 692–697 were isolated from an Okinawan specimen of Clavularia viridis.453 The Japanese octacoral Dendronephthea gigantea is the source of dendronesterols A 698 and B 699, the latter of which was mildly cytotoxic.454 The Caribbean gorgonian Eunicella laciniata contained a new gorgosterol derivative, gorgostane-3β,5α,6β,9α,11α-pentaol 700, as well as gorgost-5-ene-3β,9α,11α-triol 701, the stereochemistry of which was determined for the first time.455 The cytotoxic sterol peroxide, (22R,23R,24R)-5α,8α-epidioxy-22,23-methylene-24-methylcholest-6-en-3β-ol 702, was isolated from a Sinularia sp. from Taiwan.456 The Caribbean gorgonian Pseudopterogorgia americana contained two cytotoxic secosterols, 1β,3β-dihydroxy-5α,6α-epoxy-9-oxo-9,11-secogorgostan-11-ol 703 and 3β-hydroxy-5α,6α-epoxy-9-oxo-9,11-secogorgostan-11-ol 704.457 Riiseins A 705 and B 706 are cytotoxic sterol glycosides from the Brazilian octocoral Carijoa (Telesto) riisei.458
8 Bryozoans
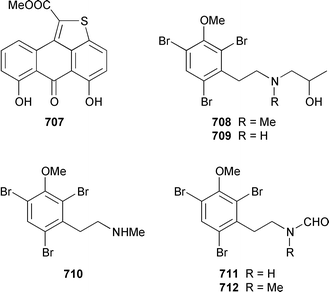
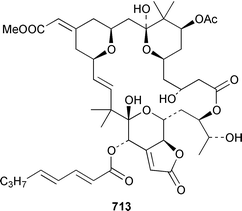
There have been no new compounds formally identified from bryozoans although three syntheses of bryozoan metabolites were reported. The structure of an orange anthrathiophene pigment 707 from a Japanese bryozoan459 was confirmed by total synthesis.460 Convalutamines A 708, C 709 and F 710, together with the yet to be reported lutamides A 711 and C 712, which are metabolites of Amathia convoluta from Florida,461,462 have all been synthesized in a straightforward manner.463 Bryostatin 3 713, which is a cytotoxic metabolite of Bugula neritina,464 has been synthesized in an enantioselective manner.4659 Molluscs
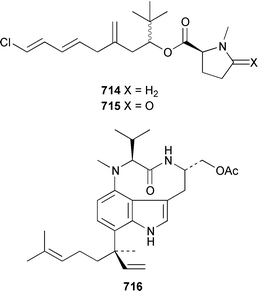
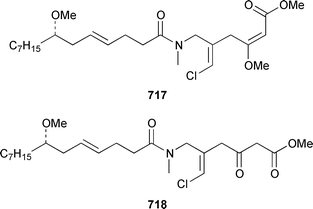
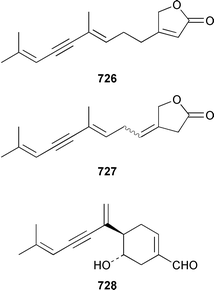
The benefits of examining different collections of shell-less marine molluscs is illustrated by the finding that a recollection of the sea hare Stylocheilus longicauda from Black Point, Oahu, contained a different suite of metabolites than had previously been obtained from specimens from Kaheohe Bay, Oahu. The new collection contained makalika ester 714, makalikone ester 715, which is mildly cytotoxic, lyngbyatoxin A acetate 716, which occurs as a 5 ∶ 1 mixture of conformers, and malyngamides O 717 and P 718, all of which are thought to be metabolites of the cyanobacterium Lyngbya majuscula.466,467 A specimen of Aplysia sp. from Madagascar contained a non-halogenated sesquiterpene 719 that is probably the rearrangement product of a known chamigrane.468 Three collections of A. dactylomela from Tenerife contained three new sesquiterpenes, puertitol B acetate 720, caespitenone 721 and 8-acetylcaespitol 722, and two new diterpenes, dactylopyranoid 723 and isopinnatol B 724, as well as a number of known metabolites of red algae of the genera Laurencia and Plocamium, the bioactivity profiles of which are reported.469The sacoglossan Elysia ornata contained kahalalide O 148, a metabolite of the green alga Bryopsis sp. on which it feeds.114 The structure of kahalalide B 725, which was isolated from both E. rufescens and Bryopsis sp.,470 has been confirmed by total synthesis.471 The sacoglossan Ascobulla ulla from the Mexican Caribbean coast contained the sesquiterpenes ascobullins A 726 and B 727, which resemble metabolites of green algae: the stereochemistry about the 3,4-double bond in ascobullin B 727 was not determined.472 (1S,6R)-Volvatellin 728, which is a metabolite of the herbivorous mollusc Volvatella sp.,473 has been synthesized from oxytoxin-1, a metabolite of the green alga Caulerpa taxifolia,474 in a highly diastereoselective manner.475
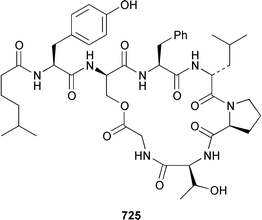
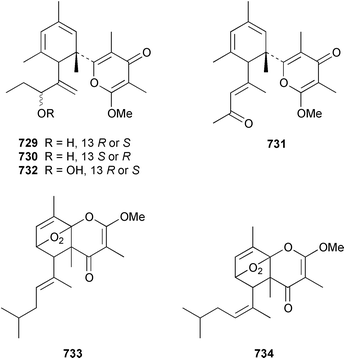
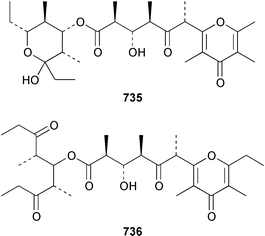
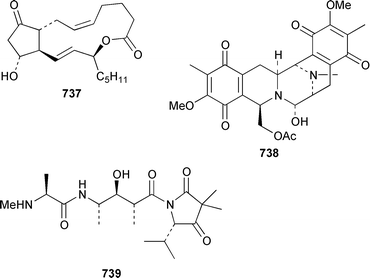
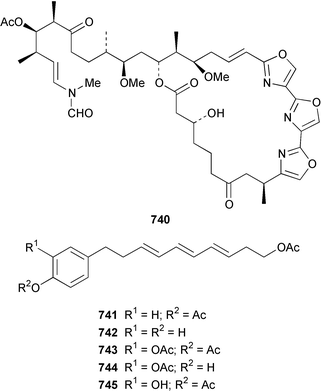
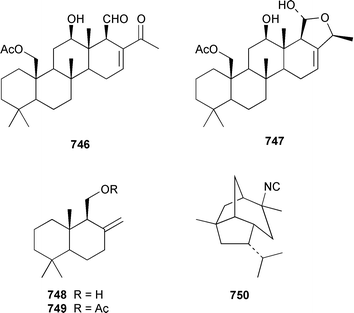
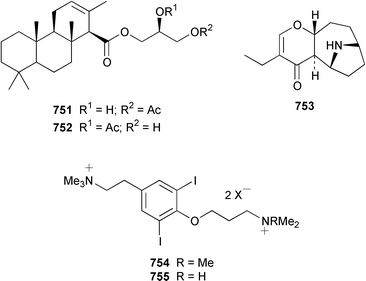
A collection of the sacoglossan Placobranchus ocellatus from the Philippines contained six additional polypropionate pyrones, tridachiapyrones G–J 729–732 and tridachiahydropyrones B 733 and C 734.476 In a paper describing the isolation of the rearranged polypropionate siserrone A 735 from Siphonaria serrata from South Africa, the natural product status of rearranged polypropionates, particularly the baconipyrones from S. baconi,477 was questioned.478 The absolute stereochemistry of (−)-baconipyrone C 736 was determined by an asymmetric total synthesis.479 Prostaglandin E2-1,15-lactone 737, which was isolated from the nudibranch Tethys fimbria,480 has been synthesized in a stereoselective manner by using a ring-closing alkyne metathesis reaction.481 The dorid nudibranch Jorunna funebris from the Mandapam coast of India, found on a sponge of the genus Oceanapia, contained the cytotoxic isoquinoline alkaloid jorumycin 738.482 The stereochemistry of janolusimide 739, which is a tripeptide neurotoxin from the nudibranch Janolus cristatus,483 has been determined by total synthesis.484 A total synthesis of the proposed structure, which was based on molecular modelling of a “dummy” metal chelate, of ulapualide A 740, which was isolated from the egg-masses of Hexabranchus sanguineus,485 suggested that the proposed relative stereochemistry differed from that of the natural product at one of the stereogenic centers in the side chain.486 Five alkyl phenols 741–745, which are proposed to be alarm pheromones of the cephalaspidean mollusc Haminoea callidegenita,487 have been synthesized by using a convergent strategy based on a Stille coupling reaction.488 Costa Rican specimens of Glossodoris sedna contained two new sesterterpenes 746, an inhibitor of phospholipase A2, and 747, while specimens of G. dalli from the same location contained different but known sesterterpenes.489 Two groups have synthesized (+)-albicanol 748 and (+)-albicanyl acetate 749, which are metabolites of the nudibranch Cadlina luteomarginata,490 by resolving key intermediates using lipase-catalyzed acetylations.491,492 A full paper on the synthesis of (±)-2-isocyanoallopupukeanane 750, which is a metabolite of Phyllidia pustulosa,493 has been published.494 A general method for the synthesis of chiral glycerides of diterpenes such as 751 and 752 from Archidoris montereyensis,495 has been reported.496
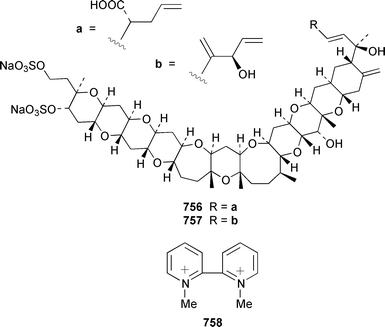
Pinnamine 753 is an alkaloidal toxin from the viscera of the bivalve Pinna muricata from Okinawa.497 Experimental details of the isolation of turbotoxins A 754 and B 755 from the gastropod Turbo marmorata have been presented.498 A new analogue of yessotoxin, carboxyyessotoxin 756, has been isolated from DSP (diarrhetic shellfish poisoning)-infested mussels Mytilus galloprovincialis from the Italian coast of the Adriatic Sea.499 The absolute configuration at C-45 in 45-hydroxyyessotoxin 757, which was isolated from shellfish, has been determined by using the modified Mosher's method.500 The bivalve Callista chione from the Aegean Sea contained 1,1′-dimethyl-[2,2′]-bipyridyldiium salt 758, which had previously been synthesized501 but had not been reported as a natural product.502
10 Tunicates (ascidians)
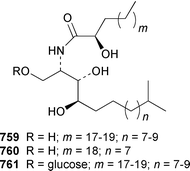
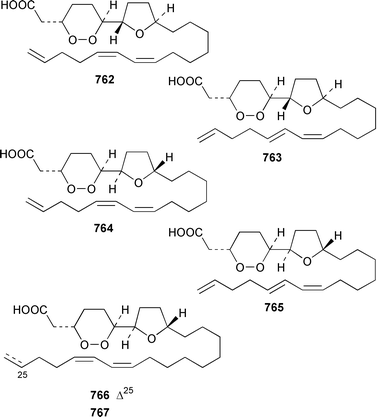
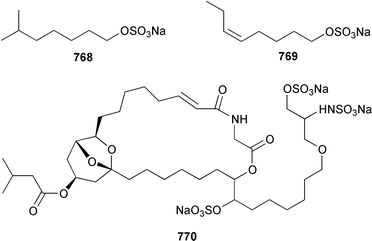
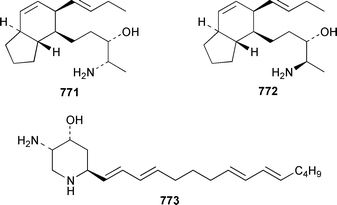
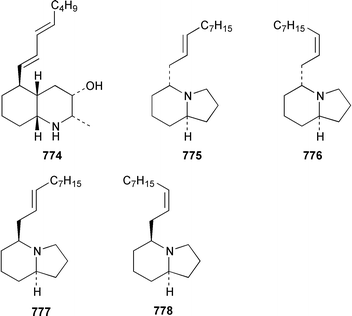
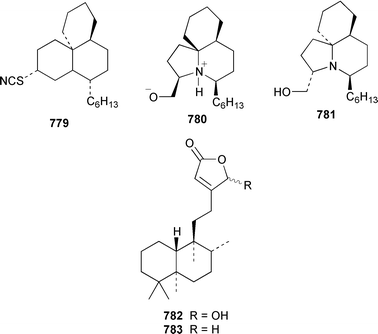
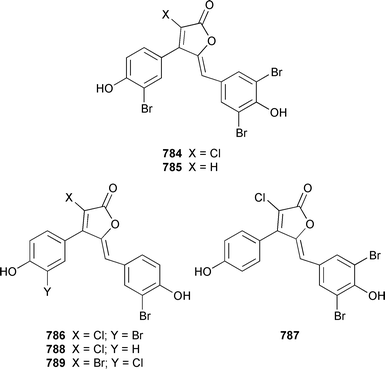
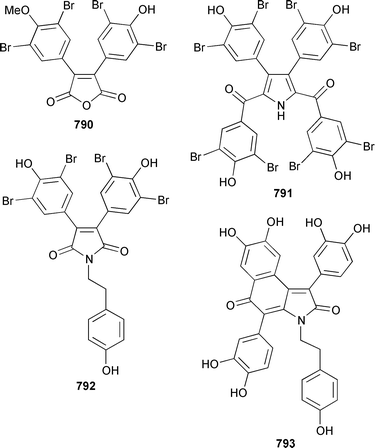
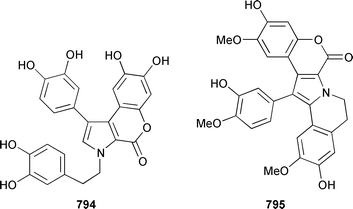
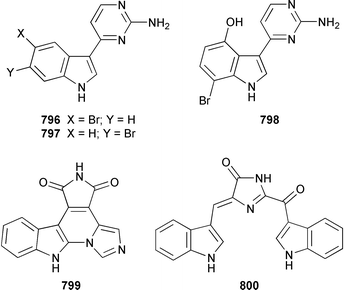
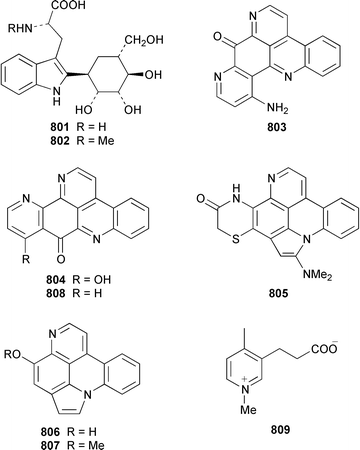
A collection of Cystodytes cf. dellechiajei from Tunisia contained a medley of sphingosines 759, of which only one sphingosine 760 was characterized, that inhibited phospholipase A2 and the corresponding ceramides 761.503 A major cyclic peroxide, stolonoxide A 762, and the minor peroxides, stolonoxides B 763, C 764 and D 765, were isolated as both the free acids and the corresponding sodium salts from the Mediterranean tunicate Stolonica socialis.504,505 Stolonic acids A 766 and B 767 were isolated as the cytotoxic metabolites of a Stolonica sp. from the Indian Ocean.506 The Mediterranean ascidian Halocynthia papillosa contained two additional cytotoxic sulfates, 6-methylheptyl sulfate 768 and (E)-oct-5-enyl sulfate 769.507 The stereochemistry of cyclodidemniserinol trisulfate 770, which was isolated from a Palauan specimen of Didemnum guttatum as an inhibitor of HIV-1 integrase, was only partially determined.508 An unidentified tunicate of the family Polyclinidae from Ukeshima Island, Japan, contained the cytotoxic amino alcohols, amainols A 771 and B 772.509 Pseudodistomin F 773, which is a metabolite of Pseudodistoma kanoko,510,511 has been synthesized by using a general method to construct 2,4,5-trisubstituted piperidines from enantiopure β-amino esters.512 (−)-Lepadin B 774, which was isolated from Clavelina lepadiformis and the flatworm Prostheceraeus villatus,513 has been synthesized in an enantioselective manner.514 Two groups515,516 have synthesized piclavines A1 775 and A2 776, which are metabolites of Clavelina picta,517 and one of these groups has also prepared piclavines A3 777 and A4 778.516 The structure of fascicularin 779, which was isolated from Nephteis fascicularis,518 has been confirmed and the structure of lepadoformine, a metabolite of C. lepadiformis,519 has been revised from 780 to 781 as a result of the total synthesis of their racemates by using a stereocontrolled intramolecular Diels–Alder reaction.520,521 Dytesinins A 782 and B 783 are cyclopropane-containing diterpenes from an Okinawan species of Cystodytes.522Six additional brominated aromatic pigments, rubrolides I–N 784–789, some of which showed significant cytotoxicity, were isolated as minor constituents of Synoicum blochmanni from Spain.523 The related metabolites prepolycitrin A 790 and polycitone B 791 were isolated as minor metabolites of Polycitor africanus from Madagascar.524 The structure of polycitrin B 792, which is an alkaloid isolated from Polycitor sp.,525 has been confirmed by total synthesis.526 Ningalin C 793, which was isolated from an unidentified Didemnum sp.,527 has been synthesized in five steps with an overall yield of 19%.247 The related metabolite, ningalin B 794, was synthesized and shown to be a multi-drug resistant reversal agent.528 Lamellarin L 795, which is a metabolite of a Didemnum sp.,529 has been synthesized by using a biomimetic strategy.530 Meridianins C–E 796–798, which are metabolites of Aplidium meridianum,531 have been synthesized in good yields from appropriately substituted N-protected 3-acylindoles.532 Meridianin D 797 was synthesized by using a palladium catalyzed cross-coupling reaction as the key step.533 An improved synthesis of isogranulatimide 799, which is a G2 checkpoint inhibitor from Didemnum granulatum,534 has been reported together with the syntheses of a variety of derivatives.535 The structure of rhopaladin D 800, which is an antibacterial agent from an Okinawan Rhopalaea sp.,536 has been confirmed by total synthesis.537 The human C-glycoconjugate C2-α-D-mannosylpyranosyl-L-tryptophan 801 was isolated from Leptoclinides dubius and Pharyngodictyon cauliflos and its Nα-methyl derivative 802 was obtained from Ritterella rete.538 The structure of cystodamine, which is a pentacyclic aromatic alkaloid from the Mediterranean ascidian Cystodytes delle chiajei,539 has been revised from 803 to 804 as a result of synthetic studies.540 Cycloshermilamine D 805, which was isolated in minute amounts from Cystodytes violatinctus from the Comoros Islands, is an additional alkaloid of the pyridoacridine group that possesses a new heterocyclic ring system.541 The structures of arnoamines A 806 and B 807, which are pyridoacridine alkaloids from a Cystodytes sp. from Arno Atoll,542 have been confirmed by total synthesis.543 The syntheses of ascididemin 808 and 11-hydroxyascididemin 804, which are metabolites of Didemnum and Leptoclinides species, respectively,544,545 employ similar methodologies.546,547 As part of a structure–activity study, ascididemin 808 and a number of analogs were synthesized and evaluated in a wide range of assays.548 The N-methylpyridinium alkaloid sulcatin 809 from a Mediterranean specimen of Microcosmus vulgaris showed interesting in vitro antiproliferative activity.549
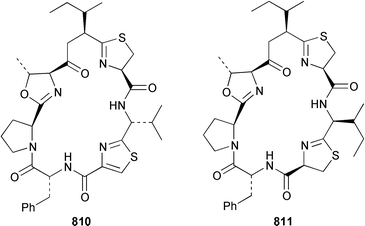
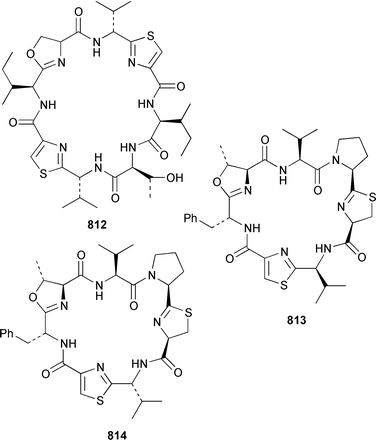
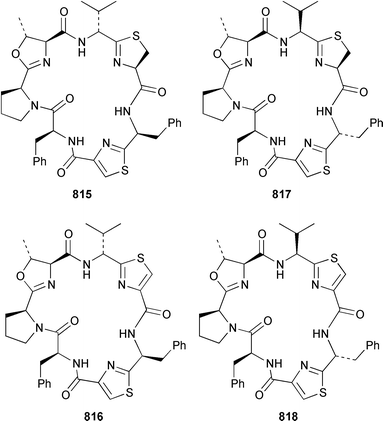
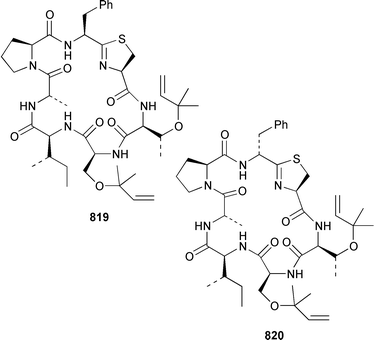
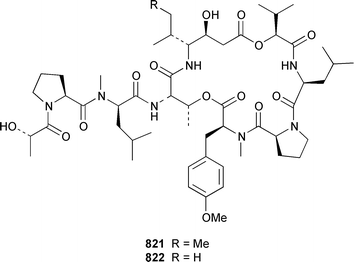
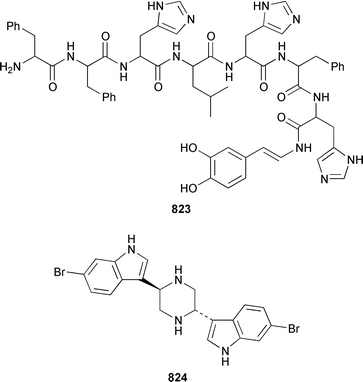
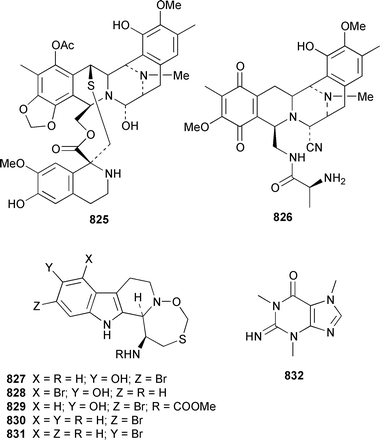
As part of a paper that described the use of NOE-restrained molecular dynamics to confirm the absolute stereochemistry of lissoclinamides from an Indonesian specimen of Lissoclinum patella, two additional cyclic peptides, lissoclinamides 9 810 and 10 811, were described.550 Prepatellamide A 812 was isolated as a minor metabolite of an Indonesian specimen of L. patella.551 The configuration of cyclodidemnamide, which was isolated from Didemnum molle,552 has been reassigned from 813 to 814 as a result of its total synthesis.553 A similar synthetic strategy allowed the configurations of lissoclinamides 4 and 5, which are metabolites of L. patella,554 to be revised from 815 and 816 to 817 and 818, respectively.555 The structure of trunkamide A, which is a metabolite of an Australian Lissoclinum sp.,556 has been revised from 819 to 820 as the result of a total synthesis.557 The cytotoxic cyclic depsipeptides tamandarins A 821 and B 822, which are related to the didemnins, were isolated from an unidentified didemnid ascidian from Brazil.558 The structure of tamandarin B 822 has been confirmed by total synthesis559 by the same group that reported the synthesis of tamandarin A 821 last year.560 Plicatamide 823 is a modified octapeptide from the blood of a San Diego Bay specimen of Styela plicata.561 The structure of 2,5-bis(6′-bromo-3′-indolyl)piperazine 824, which is a metabolite of Didemnum candidum,562 has been confirmed by employing a 4 step synthesis.283 An efficient synthesis of ecteninascidin 743 825, which is a cytotoxic agent from Ecteinascidia turbinata,563,564 from the fermentation product cyanosafracin B 826 can provide a sufficient quantity for clinical trials.565 (−)-Eudistomins C 827, E 828, F 829, K 830 and L 831, which are metabolites of Eudistoma olivaceum,566 have been synthesized from the corresponding N-hydroxytryptamines and D-cysteinal.567 1,3,7-Trimethylisoguanine 832 is a new purine derivative from a New Zealand collection of Pseudodistoma cereum.568
11 Echinoderms
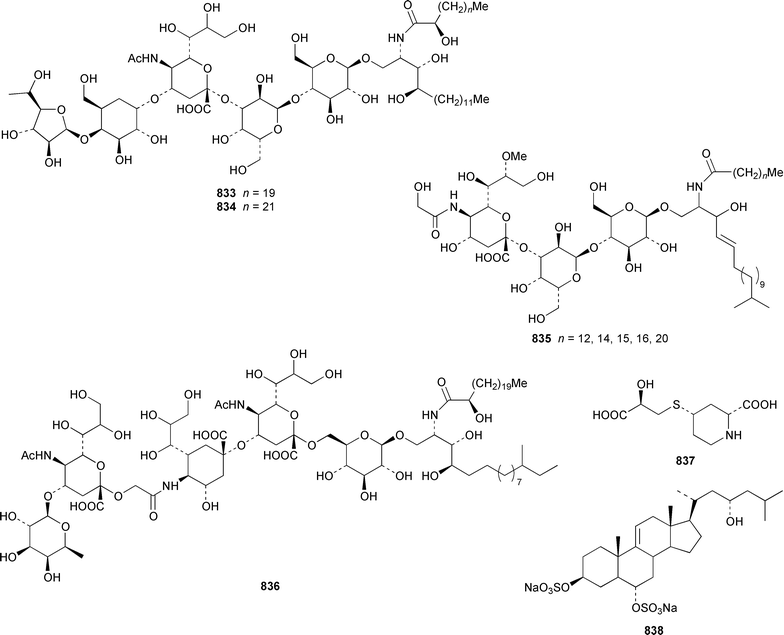
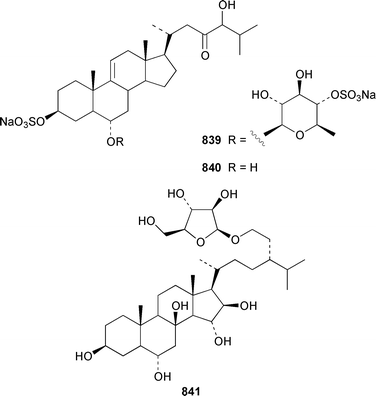
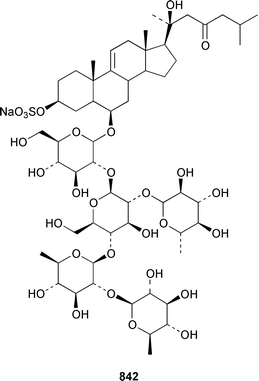
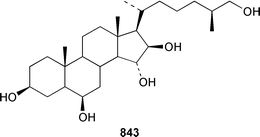
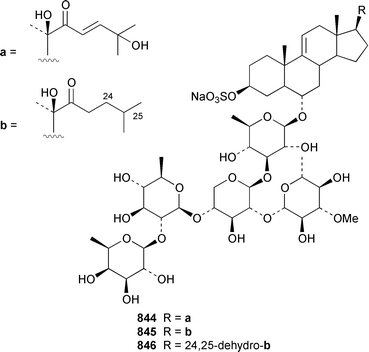
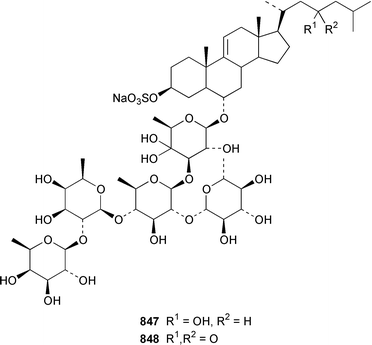
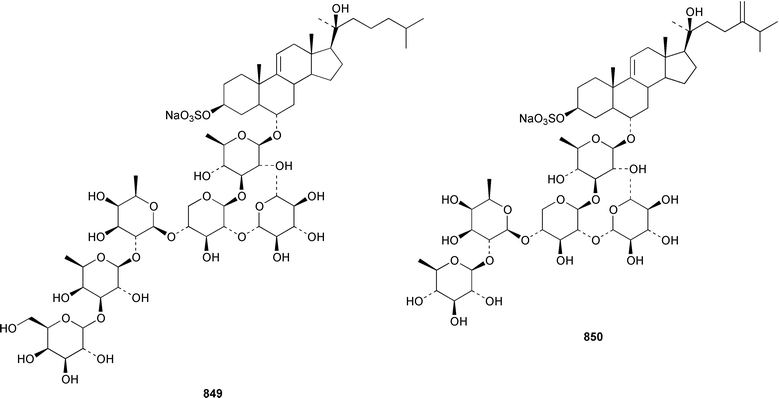
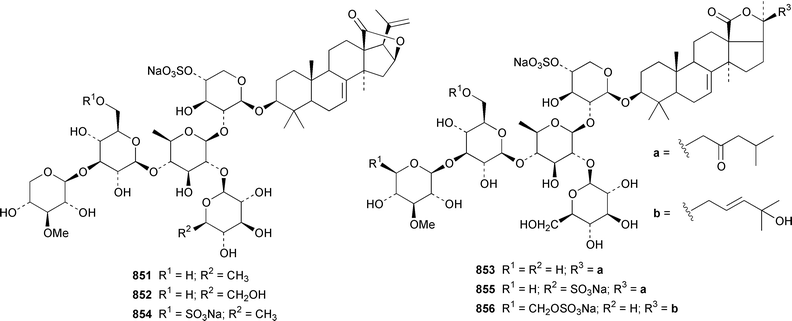
The number of papers reporting the chemistry of echinoderms is steadily declining. Two additional gangliosides, acanthagangliosides I 833 and J 834 were obtained from an Okinawan specimen of the sea star Acanthaster planci.569Linckia laevigata from Vietnam contained a ganglioside 835 with an 8-O-methyl-N-glycylneurmic acid residue.570 The same residue was found in less well defined gangliosides from the sea star Evasterias ecinosoma.571 The Japanese sea cucumber Holothuria pervicax contained the trisialo-ganglioside HPG-7 836, which showed neuritogenic activity toward the PC-12 rat pheochromocytoma cell line.572 Pucherrimine 837 is a bitter-tasting amino acid from the ovaries of the Japanese sea urchin Hemicentrotus pulcherrimus.573The metabolite of the seastar Plazaster borealis that induced an avoidance reaction in the sea urchin Strongylocentrotus nudus was identified as (3β,5α,6α,23S)-cholest-9(11)-ene-3,6,23-triol 3,6-disulfate disodium salt 838.574 A reinvestigation of the polar constituents of the seastar Aphelasterias japonica from the Russian shore of the Sea of Japan resulted in the isolation of aphelasteroside C 839 and aphelaketotriol 840 together with known saponins.575 An additional steroidal glycoside, asterosaponin P2841, has been isolated from Patiria (Asterina) pectinifera collected at the Sea of Japan.576 The Antarctic seastar Labidiaster annulatus contained labidiasteroside A 842 and (25S)-5α-cholestane-3β,6β,15α,16β,26-pentaol 843.577 The seastar Goniopecten demonstrans contained the saponins goniopectenosides A–C 844–846 that significantly inhibited settlement of the biofouling alga Hincksia irregulatus.578 Ruberosides A–D 847–850, the structures of which were elucidated by using LC-NMR-MS, are steroidal saponins from the Baltic seastar Asterias rubens.579 Studies of the sea cucumber Pentamera calcigera from the Gulf of Peter the Great have resulted in the identification of calcigerosides B 851, C1852, C2853, D1854, D2855 and E 856.580,581
12 Miscellaneous
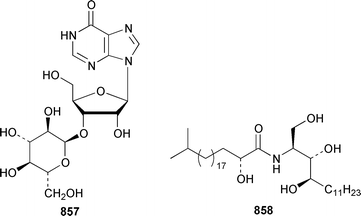
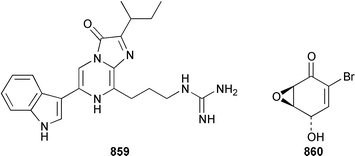
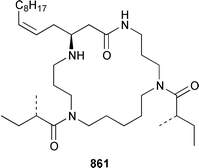
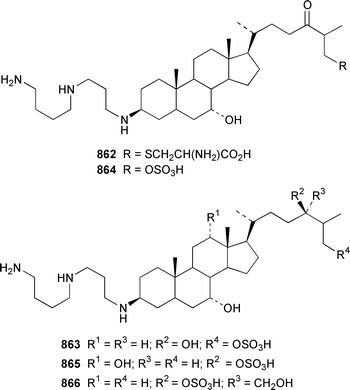
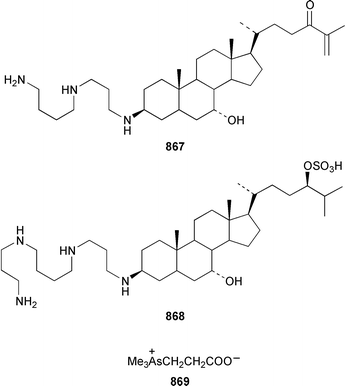
The crustacean Ligia exotica from Korea contained 3′-O-(α-D-glucosyl)inosine 857, the structure of which was confirmed by total synthesis.582 One of the sexual pheromones of the hair crab Erimacrus isenbeckii was identified as ceramide 858, which is only one of a group of homologous ceramides.583 Cypridina luciferin 859, which is involved in the bioluminescence of the crustacean Cypridina hilgendorfi,584 has been synthesized as the racemate by using Pd-mediated cross-coupling reactions.585 (+)-Bromoxone 860, which is a metabolite of the acorn worm Ptychodera sp.,586 has been prepared in an enantiopure form by using an enzymatic resolution.587 The total synthesis of lipogrammistin-A 861, which was isolated from the skin mucus of the soapfish Diploprion bifasciatum and Aulacocephalus temmincki,588 employs an efficient macrolactonization step.589 Seven additional aminosterols 862–868 have been obtained as antimicrobial agents from the liver of the dogfish shark Squalus acanthias.590 Muscle tissue of the reef fish Abudefduf vaigiensis contained significant amounts of trimethy(2-carboxyethyl)arsonium inner salt 869.591References
- D. J. Faulkner, Nat. Prod. Rep., 2001, 18, 1 RSC.
- M. G. Haygood, E. W Schmidt, S. K Davidson and D. J. Faulkner, in Molecular Marine Microbiology, ed. D. H. Bartlett, Horizon Scientific Press, 2000, pp. 61–84 Search PubMed.
- B. S. Moore, Nat. Prod. Rep., 1999, 16, 653 RSC.
- J. Kobayasi and M. Ishibashi, in Comprehensive Natural Products Chemistry, vol. 8, ed. K. Mori, Elsevier, Oxford, 1999, pp. 415–649 Search PubMed.
- P. R. Jensen and W. Fenical, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 6–29 Search PubMed.
- Y. Shimizu, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 30–45 Search PubMed.
- M. Kobayashi, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 46–58 Search PubMed.
- K. Yamada, M. Ojika, H. Kigoshi and K. Suenaga, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 59–73 Search PubMed.
- B. M. Olivera, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 74–85 Search PubMed.
- T. Natori, K. Motoki, T. Higa and Y. Koezuka, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 86–97 Search PubMed.
- M. Kuramoto, K. Yamaguchi, T. Tsuji and D. Uemura, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 98–106 Search PubMed.
- D. J. Faulkner, M. K. Harper, M. G. Haygood, C. E. Salomon and E. W. Schmidt, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 107–119 Search PubMed.
- D. Mendola in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 120–133 Search PubMed.
- J. B. Hart, R. E. Lill, S. J. H. Hickford, J. W. Blunt and M. H. G. Munro, in Drugs from the Sea, ed. N. Fusetani, Karger, Basel (Switzerland), 2000, pp. 134–153 Search PubMed.
- C. W. J. Chang, Prog. Chem. Org. Nat. Prod., 2000, 80, 1 Search PubMed.
- K. L. Rinehart, Med. Res. Rev., 2000, 20, 1 CrossRef CAS.
- M. Kalesse, ChemBioChem., 2000, 1, 171 CrossRef CAS.
- R. Mutter and M. Wills, Bioorg. Med. Chem., 2000, 8, 1841 CrossRef CAS.
- L. Heys, C. G. Moore and P. J. Murphy, Chem. Soc. Rev., 2000, 29, 57 RSC.
- M. Rama Rao, U. Venkatesham, K. V. Sridevi and Y. Venkateswarlu, Indian J. Chem., Sect. B Org. Chem. Incl. Med. Chem., 2000, 39, 723 Search PubMed.
- J. J. Fernández, M. L. Souto and M. Norte, Nat. Prod. Rep., 2000, 17, 235 RSC.
- M. Murata and T. Yasumoto, Nat. Prod. Rep., 2000, 17, 293 RSC.
- C. Wicke, M. Hüners, V. Wray, M. Nimtz, U. Bilitewski and S. Lang, J. Nat. Prod., 2000, 63, 621 CrossRef CAS.
- N. I. Kalinovskaya, T. A. Kuznetsova, A. I. Kalinovsky, V. A. Denisenko, V. I. Svetashev and L. A. Romanenko, Russ. Chem. Bull., 2000, 49, 169 Search PubMed.
- J. Kobayashi, S. Mikami, H. Shigemori, T. Takao, Y. Shimonishi, S. Izuta and S. Yoshida, Tetrahedron, 1995, 51, 10487 CrossRef CAS.
- T. Shioiri and N. Irako, Tetrahedron, 2000, 56, 9129 CrossRef CAS.
- K. Kono, M. Tanaka, T. Mizuno, K. Kodama, T. Ogita and T. Kohama, J. Antibiot., 2000, 53, 753 Search PubMed.
- R. J. Capon, C. Skene, E. Lacey, J. H. Gill, J. Wicker, K. Heiland and T. Friedel, J. Nat. Prod., 2000, 63, 1682 CrossRef CAS.
- V. J. R. V. Mukku, M. Speitling, H. Laatsch and E. Helmke, J. Nat. Prod., 2000, 63, 1570 CrossRef CAS.
- C. Jaruchoktaweechai, K. Suwanborirux, S. Tanasupawatt, P. Kittakoop and P. Menasveta, J. Nat. Prod., 2000, 63, 984 CrossRef CAS.
- L. M. Cañedo, J. L. Fernández Puentes, J. Pérez Baz, X.-H. Huang and K. L. Rinehart, J. Antibiot., 2000, 53, 479 Search PubMed.
- I. H. Hardt, P. R. Jensen and W. Fenical, Tetrahedron Lett., 2000, 41, 2073 CrossRef CAS.
- I. L. C. Hernandez, M. J. L. Godinho, A. Magalhães, A. B. Schefer, A. G. Ferriera and R. G. S. Berlinck, J. Nat. Prod., 2000, 63, 664 CrossRef CAS.
- K. Yoshikawa, T. Takadera, K. Adachi, M. Nishijima and H. Sano, J. Antibiot., 1997, 50, 949 Search PubMed.
- Y. Kobayashi, Y. Nakayama and S. Yoshida, Tetrahedron Lett., 2000, 41, 1465 CrossRef CAS.
- S. De Rosa, A. De Giulio, G. Tommonaro, S. Popov and A. Kujumgiev, J. Nat. Prod., 2000, 63, 1454 CrossRef CAS.
- Z. Jiang, K. G. Boyd, A. Mearns-Spragg, D. R. Adams, P. C. Wright and J. G. Burgess, Nat. Prod. Lett., 2000, 14, 435 Search PubMed.
- J. Pérez Baz, L. M. Cañedo, J. L. Fernández Puentes and M. V. Silva Elipe, J. Antibiot., 1997, 50, 738 Search PubMed.
- D. L. Boger and S. Ichikawa, J. Am. Chem. Soc., 2000, 122, 2956 CrossRef CAS.
- L. M. Cañedo Hernández, J. A. de la Fuente Blanco, J. Pérez Baz, J. L. Fernández Puentes, F. Romero Millán, F. Espliego Vázquez, R. I. Fernández-Chimeno and D. García Grávalos, J. Antibiot., 2000, 53, 895 Search PubMed.
- T. Kagata, H. Shigemori, Y. Mikami and J. Kobayashi, J. Nat. Prod., 2000, 63, 886 CrossRef CAS.
- C. J. Smith, D. Abbanat, V. S. Bernan, W. M. Maiese, M. Greenstein, J. Jompa, A. Tahir and C. M. Ireland, J. Nat. Prod., 2000, 63, 142 CrossRef CAS.
- R. A. Edrada, V. Wray, A. Berg, U. Gräfe, Sudarsono, G. Brauers and P. Proksch, Z. Naturforsch., C: Biosci., 2000, 55, 218 Search PubMed.
- A. Numata, M. Iritani, T. Yamada, K. Minoura, E. Matsumura, T. Yamori and T. Tsuruo, Tetrahedron Lett., 1997, 38, 8215 CrossRef CAS.
- M. Ono, H. Nakamura, F. Konno and H. Akita, Tetrahedron: Asymmetry, 2000, 11, 2753 CrossRef CAS.
- M. Varoglu and P. Crews, J. Nat. Prod., 2000, 63, 41 CrossRef CAS.
- J. Malmstrøm, C. Christophersen and J. C. Frisvad, Phytochemistry, 2000, 54, 301 CrossRef CAS.
- G. Brauers, R. A. Edrada, R. Ebel, P. Proksch, V. Wray, A. Berg, U. Gräfe, C. Schächtele, F. Totzke, G. Finkenzeller, D. Marme, J. Kraus, M. Münchbach, M. Michel, G. Bringmann and K. Schaumann, J. Nat. Prod., 2000, 63, 739 CrossRef CAS.
- M. Namikoshi, H. Kobayashi, T. Yoshimoto and S. Meguro, Chem. Lett., 2000, 308 CrossRef CAS.
- M. Namikoshi, H. Kobayashi, T. Yoshimoto, S. Meguro and K. Akano, Chem. Pharm. Bull., 2000, 48, 1452 Search PubMed.
- K. Komatsu, H. Shigemori, Y. Mikami and J. Kobayashi, J. Nat. Prod., 2000, 63, 408 CrossRef CAS.
- D. Laurent, G. Guella, M.-F. Roquebert, F. Farinole, I. Mancini and F. Pietra, Planta Med., 2000, 66, 63 CrossRef CAS.
- T. Amagata, Y. Usami, K. Minoura, T. Ito and A. Numata, J. Antibiot., 1998, 51, 33 Search PubMed.
- Y. Usami, T. Ikura, T. Amagata and A. Numata, Tetrahedron: Asymmetry, 2000, 11, 3711 CrossRef CAS.
- H. B. Mereyala, M. Joe and R. R. Gadikota, Tetrahedron: Asymmetry, 2000, 11, 4071 CrossRef CAS.
- A. Numata, T. Amagata, K. Minoura and T. Ito, Tetrahedron Lett., 1997, 38, 5675 CrossRef CAS.
- T. Amagata, M. Doi, T. Ohta, K. Minoura and A. Numata, J. Chem. Soc., Perkin Trans. 1, 1998, 3585 RSC.
- M. K. Gurjar and P. Bhaket, Heterocycles, 2000, 53, 143 Search PubMed.
- T. S. Bugni, D. Abbanat, V. S. Bernan, W. M. Maiese, M. Greenstein, R. M. Van Wagoner and C. M. Ireland, J. Org. Chem., 2000, 65, 7195 CrossRef CAS.
- S. S. Afiyatullov, T. A. Kuznetsova, V. V. Isakov, M. V. Pivkin, N. G. Prokof'eva and G. B. Elyakov, J. Nat. Prod., 2000, 63, 848 CrossRef CAS.
- M. K. Renner, P. R. Jensen and W. Fenical, J. Org. Chem., 2000, 65, 4843 CrossRef CAS.
- M. Cueto, P. R. Jensen and W. Fenical, Phytochemistry, 2000, 55, 223 CrossRef CAS.
- G. N. Belofsky, P. R. Jensen and W. Fenical, Tetrahedron Lett., 1999, 40, 2913 CrossRef CAS.
- Y. Lee and R. B. Silverman, Org. Lett., 2000, 2, 3743 CrossRef CAS.
- K. Suenaga, S. Aoyama, W. Xi, H. Arimoto, K. Yamaguchi, K. Yamada, T. Tsuji, A. Yamada and D. Uemura, Heterocycles, 2000, 52, 1033 Search PubMed.
- C. Osterhage, R. Kaminsky, G. M. König and A. D. Wright, J. Org. Chem., 2000, 65, 6412 CrossRef CAS.
- G. N. Belofsky, M. Anguera, P. R. Jensen, W. Fenical and M. Köck, Chem. Eur. J., 2000, 6, 1355 CrossRef CAS.
- A. Numata, C. Takahashi, T. Matsushita, T. Miyamoto, K. Kawai, Y. Usami, E. Matsumura, M. Inoue, H. Ohishi and T. Shingu, Tetrahedron Lett., 1992, 33, 1621 CrossRef CAS.
- B. B. Snider and H. Zeng, Org. Lett., 2000, 2, 4103 CrossRef CAS.
- H. Wang and A. Ganesan, J. Org. Chem., 2000, 65, 1022 CrossRef CAS.
- Y. Lin, Z. Shao, G. Jiang, S. Zhou, J. Cai, L. L. P. Vrijmoed and E. B. G. Jones, Tetrahedron, 2000, 56, 9607 CrossRef CAS.
- M. Wu, T. Okino, L. M. Nogle, B. L. Marquez, R. T. Williamson, N. Sitachitta, F. W. Berman, T. F. Murray, K. McGough, R. Jacobs, K. Colsen, T. Asano, F. Yokoyawa, T. Shioiri and W. H. Gerwick, J. Am. Chem. Soc., 2000, 122, 12041 CrossRef CAS.
- I. P. Singh, K. E. Milligan and W. H. Gerwick, J. Nat. Prod., 1999, 62, 1333 CrossRef CAS.
- R. M. Kanada, T. Taniguchi and K. Ogasawara, Synlett, 2000, 1019 CAS.
- D. G. Nagle, P. U. Park and V. J. Paul, Tetrahedron Lett., 1997, 38, 6969 CrossRef CAS.
- S. Ribe, R. K. Kondru, D. N. Beratan and P. Wipf, J. Am. Chem. Soc., 2000, 122, 4608 CrossRef CAS.
- J. Orjala, D. G. Nagle, V. L. Hsu and W. H. Gerwick, J. Am. Chem. Soc., 1995, 117, 8281 CrossRef CAS.
- F. Yokokawa, H. Fujiwara and T. Shioiri, Tetrahedron, 2000, 56, 1759 CrossRef CAS.
- T. Tan, T. Okino and W. H. Gerwick, J. Nat. Prod., 2000, 63, 952 CrossRef.
- K. E. Milligan, B. Márquez, R. T. Williamson, M. Davies-Coleman and W. H. Gerwick, J. Nat. Prod., 2000, 63, 965 CrossRef CAS.
- Y. Kan, B. Sakamoto, T. Fujita and H. Nagai, J. Nat. Prod., 2000, 63, 1599 CrossRef CAS.
- D. G. Nagle, Y.-D. Zhou, P. U. Park, V. J. Paul, I. Rajbhandari, C. J. G. Duncan and D. S. Pasco, J. Nat. Prod., 2000, 63, 1431 CrossRef CAS.
- N. Sitachitta, B. L. Márquez, R. T. Williamson, J. Rossi, M. A. Roberts, W. H. Gerwick, V.-A. Nguyen and C. L. Willis, Tetrahedron, 2000, 56, 9103 (erratum: Tetrahedron, 2001, 57, 2915) Search PubMed.
- H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2000, 63, 1106 CrossRef CAS.
- N. Sitachitta, R. T. Williamson and W. H. Gerwick, J. Nat. Prod., 2000, 63, 197 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore, V. J. Paul and S. L. Mooberry, J. Nat. Prod., 2000, 63, 611 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2000, 63, 1437 CrossRef CAS.
- K. E. Milligan, B. L. Márquez, R. T. Williamson and W. H. Gerwick, J. Nat. Prod., 2000, 63, 1440 CrossRef CAS.
- D. Klein, J.-C. Braekman, D. Daloze, L. Hoffmann, G. Castillo and V. Demoulin, Tetrahedron Lett., 1999, 40, 695 CrossRef CAS.
- G. R. Pettit, Y. Kamano, P. Brown, D. Gust, M. Inoue and C. L. Herald, J. Am. Chem. Soc., 1982, 104, 905 CrossRef CAS.
- G. R. Pettit, Y. Kamano, C. W. Holzapfel, W. J. van Zyl, A. A. Tuinman, C. L. Herald, L. Baczynskyj and J. M. Schmidt, J. Am. Chem. Soc., 1987, 109, 7581 CrossRef CAS.
- S. S. Mitchell, D. J. Faulkner, K. Rubins and F. D. Bushman, J. Nat. Prod., 2000, 63, 279 CrossRef CAS.
- F. D. Horgen, W. Y. Yoshida and P. J. Scheuer, J. Nat. Prod., 2000, 63, 461 CrossRef CAS.
- M. Tsuda, T. Endo and J. Kobayashi, J. Org. Chem., 2000, 65, 1349 CrossRef CAS.
- T. Kubata, M. Tsuda and J. Kobayashi, Tetrahedron Lett., 2000, 41, 713 CrossRef.
- J. Kobayashi, H. Shigemori, M. Ishibashi, T. Yamasu, H. Hirota and T. Sasaki, J. Org. Chem., 1991, 56, 5221 CrossRef CAS.
- J. Kobayashi, K. Shimbo, M. Sato, M. Shiro and M. Tsuda, Org. Lett., 2000, 2, 2805 CrossRef CAS.
- M. Ishibashi, M. Takahashi and J. Kobayashi, J. Org. Chem., 1995, 60, 6062 CrossRef CAS.
- M. Ishibashi and J. Kobayashi, Heterocycles, 1997, 44, 543 Search PubMed.
- D. R. Williams, B. J. Myers and L. Mi, Org. Lett., 2000, 2, 945 CrossRef CAS.
- T. Kubota, M. Tsuda, M. Takahashi, M. Ishibashi, S. Oka and J. Kobayashi, Chem. Pharm. Bull., 2000, 48, 1447 Search PubMed.
- C. O. Miles, A. L. Wilkins, D. J. Stirling and A. L. MacKenzie, J. Agric. Food Chem., 2000, 48, 1373 CrossRef CAS.
- T. Yasumoto, T. Igarashi, A.-M. Legrand, P. Cruchet, M. Chinain, T. Fujita and H. Naoki, J. Am. Chem. Soc., 2000, 122, 4988 CrossRef CAS.
- H. Nagai, M. Murata, K. Torigoe, M. Sataka and T. Yasumoto, J. Org. Chem., 1992, 57, 5448 CrossRef CAS.
- A. Morohashi, M. Satake, H. Nagai, Y. Oshima and T. Yasumoto, Tetrahedron, 2000, 56, 8995 CrossRef CAS.
- S. T. Belt, G. Allard, G. Massé, J.-M. Robert and S. Rowland, Chem. Commun., 2000, 501 RSC.
- R. Wang, Y. Shimizu, J. Rios Steiner and J. Clardy, J. Chem. Soc., Chem. Commun., 1993, 397 RSC.
- H. Miyaoka, M. Tamura and Y. Yamada, Tetrahedron, 2000, 56, 8083 CrossRef CAS.
- J.-L. Giner and X. Li, Tetrahedron, 2000, 56, 9575 CrossRef CAS.
- B. Sakamoto, Y. Hokama, F. D. Horgen, P. J. Scheuer, Y. Kan and H. Nagai, J. Phycol., 2000, 36, 924 CrossRef CAS.
- D. Iliopoulou, C. Vagias, C. Harvala and V. Roussis, Nat. Prod. Lett., 2000, 14, 373 Search PubMed.
- J. T. Handley and A. J. Blackman, Aust. J. Chem., 2000, 53, 67 CrossRef CAS.
- N. E. Awad, Phytother. Res., 2000, 14, 641 CrossRef CAS.
- F. D. Horgen, D. B. delos Santos, G. Goetz, B. Sakamoto, Y. Kan, H. Nagai and P. J. Scheuer, J. Nat. Prod., 2000, 63, 152 CrossRef CAS.
- F.-J. Marner, B. Müller and L. Jaenicke, Z. Naturforsch., C: Biosci., 1984, 39, 689 Search PubMed.
- C. Hertweck and W. Boland, J. Org. Chem., 2000, 65, 2458 CrossRef CAS.
- J. A. Pettus and R. E. Moore, J. Chem. Soc., Chem. Commun., 1970, 1093 RSC.
- T. Itoh, H. Inoue and S. Emoto, Bull. Chem. Soc. Jpn., 2000, 73, 409 CrossRef CAS.
- L. M. Murray, R. A. Barrow and R. J. Capon, Aust. J. Chem., 1991, 44, 843 CAS.
- C. G. García, M. A. Solar and V. S. Martín, Tetrahedron Lett., 2000, 41, 4127 CrossRef CAS.
- P. Sampli, C. Tsitsimpikou, C. Vagias, C. Harvala and V. Roussis, Nat. Prod. Lett., 2000, 14, 365 Search PubMed.
- W. Fenical, J. J. Sims, D. Squatrito, R. M. Wing and P. Radlick, J. Org. Chem., 1973, 38, 2383 CrossRef CAS.
- J. Schröder, C. Magg and K. Siefert, Tetrahedron Lett., 2000, 41, 5469 CrossRef CAS.
- Y. C. Shen, P. I. Tsai, W. Fenical and M. E. Hay, Phytochemistry, 1993, 52, 71.
- Y. Li, H. Yuan, B. Lu, Y. Li and D. Teng, J. Chem. Res. (S), 2000, 530 Search PubMed.
- J. S. Edmonds, Bioorg. Med. Chem. Lett., 2000, 10, 1105 CrossRef CAS.
- Z. D. Jiang, S. O. Ketchum and W. H. Gerwick, Phytochemistry, 2000, 53, 129 CrossRef CAS.
- D. G. Nagle and W. H. Gerwick, J. Org. Chem., 1994, 59, 7227 CrossRef CAS.
- C. Barloy-Da Silva, A. Benkouider and P. Pale, Tetrahedron Lett., 2000, 41, 3077 CrossRef.
- M. B. Govenkar and S. Wahidulla, Phytochemistry, 2000, 54, 979 CrossRef CAS.
- C. Flodin and F. B. Whitfield, Phytochemistry, 2000, 53, 77 CrossRef CAS.
- J. S. Choi, H. J. Park, H. A. Jung, H. Y. Chung, J. H. Jung and W. C. Choi, J. Nat. Prod., 2000, 63, 1705 CrossRef CAS.
- G. Guella, I. Mancini, A. Öztunç and F. Pietra, Helv. Chim. Acta, 2000, 83, 336 CrossRef CAS.
- E. Kurosawa, A. Kukusawa and T. Irie, Tetrahedron Lett., 1972, 2121 CrossRef CAS.
- T. Martín and V. S. Martín, Tetrahedron Lett., 2000, 41, 2503 CrossRef CAS.
- A. Fukuzawa, Y. Takasugi and A. Murai, Tetrahedron Lett., 1991, 32, 5597 CrossRef CAS.
- A. Fukuzawa and E. Kurosawa, Tetrahedron Lett., 1979, 2797 CrossRef CAS.
- M. T. Crimmins and E. A. Tabet, J. Am. Chem. Soc., 2000, 122, 5473 CrossRef CAS.
- L. T. Tan, R. T. Williamson, W. H. Gerwick, K. S. Watts, K. McGough and R. Jacobs, J. Org. Chem., 2000, 65, 419 CrossRef CAS.
- M. Yotsu-Yamashita, R. L. Haddock and T. Yasumoto, J. Am. Chem. Soc., 1993, 115, 1147 CrossRef CAS.
- L. A. Paquette, L. Barriault, D. Pissarnitski and J. N. Johnston, J. Am. Chem. Soc., 2000, 122, 619 CrossRef CAS.
- J. Jongaramruong and A. J. Blackman, J. Nat. Prod., 2000, 63, 272 CrossRef CAS.
- R. W. Fuller, J. H. Cardellina II, J. Jurek, P. J. Scheuer, B. Alvarado-Lindner, M. McGuire, G. N. Gray, J. R. Steiner, J. Clardy, E. Menez, R. H. Shoemaker, D. J. Newman, K. M. Snader and M. R. Boyd, J. Med. Chem., 1994, 37, 4407 CrossRef CAS.
- T. Sotokawa, T. Noda, S. Pi and M. Hirama, Angew. Chem., Int. Ed., 2000, 39, 3430 CrossRef CAS.
- C. O. Oh, J. W. Han, J. S. Kim, S. Y. Um, H. H. Jung, W. Y. Jang and H. S. Won, Tetrahedron Lett., 2000, 41, 8365–8369 CrossRef CAS.
- T. Irie, T. Suzuki, Y. Yasunari, E. Kurosawa and T. Masamune, Tetrahedron, 1969, 25, 459 CrossRef CAS.
- G. Guella and F. Pietra, Helv. Chim. Acta, 2000, 83, 2946 CrossRef CAS.
- M. Kuniyoshi, M. S. Marma, T. Higa, G. Bernardinelli and C. W. Jefford, Chem. Commun., 2000, 1155 RSC.
- L. Ktari, A. Blond and M. Guyot, Bioorg. Med. Chem. Lett., 2000, 10, 2563 CrossRef CAS.
- F. D. Horgen, B. Sakamoto and P. J. Scheuer, J. Nat. Prod., 2000, 63, 210 CrossRef CAS.
- T. Suzuki, M. Suzuki, A. Furusaki, T. Matsumoto, A. Kato, Y. Imanaka and E. Kurosawa, Tetrahedron Lett., 1985, 26, 1329 CrossRef CAS.
- I. C. González and C. J. Forsyth, J. Am. Chem. Soc., 2000, 122, 9099 CrossRef CAS.
- N. M. Carballeira and M. Pagán, J. Nat. Prod., 2000, 63, 666 CrossRef CAS.
- K. Watanabe, Y. Tsuda, M. Iwashima and K. Iguchi, J. Nat. Prod., 2000, 63, 258 CrossRef CAS.
- H. Niwa, K. Wakamatsu and K. Yamada, Tetrahedron Lett., 1989, 30, 4543 CrossRef CAS.
- Y. Takemoto, Y. Baba, G. Saha, S. Nakao, C. Iwata, T. Tanaka and T. Ibuka, Tetrahedron Lett., 2000, 41, 3653 CrossRef CAS.
- Z. Zeng, Redai Haiyang, 2000, 19, 86 Search PubMed.
- V. Costantino, E. Fattorusso and A. Mangoni, Tetrahedron, 2000, 56, 5953 CrossRef CAS.
- V. Costantino, E. Fattorusso, A. Mangoni, M. Di Rosa and A. Ionaro, J. Am. Chem. Soc., 1997, 119, 12465 CrossRef CAS.
- K. C. Nicolaou, J. Li and G. Zenke, Helv. Chim. Acta, 2000, 83, 1977 CrossRef CAS.
- T. N. Makarieva, V. A. Denisenko and V. A. Stonik, Tetrahedron Lett., 1989, 30, 6581 CrossRef CAS.
- G. M. Nicolas, T. W. Hong, T. F. Molinski, M. L. Lerch, M. T. Cancilla and C. B. Lebrilla, J. Nat. Prod., 1999, 62, 1678 CrossRef CAS.
- T. F. Molinski, T. N. Makarieva and V. A. Stonik, Angew. Chem., Int. Ed., 2000, 39, 4076 CrossRef CAS.
- G. M. Nicholas and T. F. Molinski, J. Am. Chem. Soc., 2000, 122, 4011 CrossRef CAS.
- C. Devijver, M. Salmoun, D. Daloze, J. C. Braekman, W. H. De Weerdt, M. J. De Kluijver and R. Gomez, J. Nat. Prod., 2000, 63, 978 CrossRef CAS.
- Y.-W. Guo and E Trivellone, J. Asian Nat. Prod. Res., 2000, 2, 251 Search PubMed.
- S. Matsunaga, Y. Okada, N. Fusetani and R. W. M. van Soest, J. Nat. Prod., 2000, 63, 690 CrossRef CAS.
- K. Watanabe, Y. Tsuda, Y. Yamane, H. Takahashi, K. Iguchi, H. Naoki, T. Fujita and R. W. M. Van Soest, Tetrahedron Lett., 2000, 41, 9271 CrossRef CAS.
- Y.-C. Shen and C. V. S. Prakash, J. Nat. Prod., 2000, 63, 1686 CrossRef CAS.
- D. T. A. Youssef, W. Y. Yoshida, M. Kelly and P. J. Scheuer, J. Nat. Prod., 2000, 63, 1406 CrossRef CAS.
- S. Aoki, K. Matsui, K. Tanaka, R. Satari and M. Kobayashi, Tetrahedron, 2000, 56, 9945 CrossRef CAS.
- M. A. Rashid, K. R. Gustafson and M. R. Boyd, Nat. Prod. Lett., 2000, 14, 387 Search PubMed.
- L. Chill, A. Miroz and Y. Kashman, J. Nat. Prod., 2000, 63, 523 CrossRef CAS.
- E. Fattorusso, O. Tagliatatela-Scafati, M. Di Rosa and A. Ianaro, Tetrahedron, 2000, 56, 7959 CrossRef CAS.
- S. Aoki, K. Higuchi, Y. Ye, R. Satari and M. Kobayashi, Tetrahedron, 2000, 56, 1833 CrossRef CAS.
- J. S. Simpson, M. J. Garson, J. W. Blunt, M. H. G. Munro and J. N. A. Hooper, J. Nat. Prod., 2000, 63, 704 CrossRef CAS.
- L. M. West, P. T. Northcote, K. A. Hood, J. H. Miller and M. J. Page, J. Nat. Prod., 2000, 63, 707 CrossRef CAS.
- N. B. Perry, J. W. Blunt, M. H. G. Munro and A. M. Thompson, J. Org. Chem., 1990, 55, 223 CrossRef CAS.
- N. Fusetani, T. Sugawara and S. Matsunaga, J. Org. Chem., 1992, 57, 3828 CrossRef CAS.
- W. R. Roush and L. A. Pfiefer, Org. Lett., 2000, 2, 859 CrossRef CAS.
- P. Kocienski, R. Narquizian, P. Raubo, C. Smith, L. J. Farrugia, K. Muir and F. T. Boyle, J. Chem. Soc., Perkin Trans. 1, 2000, 2357 RSC.
- L. M. West, P. T. Northcote and C. N. Battershill, J. Org. Chem., 2000, 65, 445 CrossRef CAS.
- C. L. Cantrell, K. R. Gustafson, M. R. Cecere, L. K. Pannell and M. R. Boyd, J. Am. Chem. Soc., 2000, 122, 8825 CrossRef CAS.
- Y. Nakao, T. Maki, S. Matsunaga, R. W. M. van Soest and N. Fusetani, Tetrahedron, 2000, 56, 8977 CrossRef CAS.
- A. B. Smith, III, T. J. Beauchamp, M. J. LaMarche, M. D. Kaufman, Y. Qiu, H. Arimoto, D. R. Jones and K. Kobayashi, J. Am. Chem. Soc., 2000, 122, 8654 CrossRef.
- S. P. Gunasekera, M. Gunasekera, R. E. Longley and G. K. Schulte, J. Org. Chem., 1990, 55, 4912 [erratum J. Org. Chem., 1991, 56, 1346] Search PubMed.
- I. Paterson, G. J. Florence, K. Gerlach and J. P. Scott, Angew. Chem., Int. Ed., 2000, 39, 377 CrossRef.
- I. Paterson and G. J. Florence, Tetrahedron Lett., 2000, 41, 6935 CrossRef CAS.
- S. Tsukamoto, S. Takeuchi, M. Ishibashi and J. Kobayashi, J. Org. Chem., 1992, 57, 5255 CrossRef CAS.
- M. Ishibashi, S. Takeuchi and J. Kobayashi, Tetrahedron Lett., 1993, 34, 3749 CrossRef CAS.
- S. Al-Busafi and R. C. Whitehead, Tetrahedron Lett., 2000, 41, 3467 CrossRef CAS.
- S. Takeuchi, M. Ishibashi and J. Kobayashi, J. Org. Chem., 1994, 59, 3712 CrossRef CAS.
- D. Ma and H. Sun, Tetrahedron Lett., 2000, 41, 1947 CrossRef CAS.
- K. L. Erickson, J. A. Beutler, J. H. Cardellina II and M. R. Boyd, J. Org. Chem., 1997, 62, 8188 CrossRef CAS.
- Y. Wu, L. Esser and J. K. De Brabander, Angew. Chem., Int. Ed., 2000, 39, 4308 CrossRef CAS.
- Y. Wu, O. R. Seguil and J. K. De Brabander, Org. Lett., 2000, 2, 4241 CrossRef CAS.
- P. Crews, Y. Kakou and E. Quiñoà, J. Am. Chem. Soc., 1988, 110, 4365 CrossRef CAS.
- H. Sugiyama, F. Yokokawa and T. Shioiri, Org. Lett., 2000, 2, 2149 CrossRef CAS.
- T. Ichiba, W. Y. Yoshida, P. J. Scheuer and T. Higa, J. Am. Chem. Soc., 1991, 113, 3173 CrossRef CAS.
- F. Yokokawa, T. Asano and T. Shioiri, Org. Lett., 2000, 3, 4169 CrossRef CAS.
- M. D'Ambrosio, A. Guerriero, C. Debitus and F. Pietra, Helv. Chim. Acta, 1996, 79, 51 CrossRef CAS.
- K. R. Hornberger, C. L. Hamblett and J. L. Leighton, J. Am. Chem. Soc., 2000, 122, 12894 CrossRef CAS.
- P. T. Northcote, J. W. Blunt and M. H. G. Munro, Tetrahedron Lett., 1991, 32, 6411 CrossRef CAS.
- M. J. Remuiñán and G. Pattenden, Tetrahedron Lett., 2000, 41, 7367 CrossRef CAS.
- D. G. Corley, R. Herb, R. E. Moore, P. J. Scheuer and V. J. Paul, J. Org. Chem., 1988, 53, 3644 CrossRef CAS.
- E. Quiñoà, Y. Kakou and P. Crews, J. Org. Chem., 1988, 53, 3642 CrossRef CAS.
- A. K. Ghosh and Y. Wang, J. Am. Chem. Soc., 2000, 122, 11027 CrossRef CAS.
- P. A. Searle and T. F. Molinski, J. Am. Chem. Soc., 1995, 117, 8126 CrossRef CAS.
- D. A. Evans and D. M. Fitch, Angew. Chem., Int. Ed., 2000, 39, 2536 CrossRef CAS.
- D. A. Evans, D. M. Fitch, T. E. Smith and V. J. Cee, J. Am. Chem. Soc., 2000, 122, 10033 CrossRef CAS.
- N. Fusetani, K. Yasumuro, S. Matsunaga and K. Hashimoto, Tetrahedron Lett., 1989, 30, 2809 CrossRef CAS.
- P. Liu and J. S. Panek, J. Am. Chem. Soc., 2000, 122, 1235 CrossRef CAS.
- J. S. Panek and P. Liu, J. Am. Chem. Soc., 2000, 122, 11090 CrossRef CAS.
- J. B. MacMillan and T. F. Molinski, J. Nat. Prod., 2000, 63, 155 CrossRef CAS.
- J. B. MacMillan, E. K. Trousdale and T. F. Molinski, Org. Lett., 2000, 2, 2721 CrossRef CAS.
- R. Sakai, H. Kamiya, M. Murata and K. Shimamoto, J. Am. Chem. Soc., 1997, 119, 4112 CrossRef CAS.
- B. B. Snider and N. A. Hawryluk, Org. Lett., 2000, 2, 635 CrossRef CAS.
- M. Sasaki, T. Koike, R. Sakai and K. Tachibana, Tetrahedron Lett., 2000, 41, 3923 CrossRef CAS.
- H. Masaki, J. Maeyama, K. Kamada, T. Esumi, Y. Iwabuchi and S. Hatakeyama, J. Am. Chem. Soc., 2000, 122, 5216 CrossRef CAS.
- Y. Nakao, M. Fujita, K. Warabi, S. Matsunaga and N. Fusetani, J. Am. Chem. Soc., 2000, 122, 10462 CrossRef CAS.
- M. A. Rashid, K. R. Gustafson, J. L. Boswell and M. R. Boyd, J. Nat. Prod., 2000, 63, 956 CrossRef CAS.
- A. Qureshi, P. L. Colin and D. J. Faulkner, Tetrahedron, 2000, 56, 3679 CrossRef CAS.
- I. Erdoğan, J. Tanaka and T. Higa, FABAD J. Pharm. Sci., 2000, 25, 7 Search PubMed.
- M. C. Roy, I. I. Ohtani, T. Ichiba, J. Tanaka, R. Satari and T. Higa, Tetrahedron, 2000, 56, 9079 CrossRef CAS.
- M. Kobayashi, M. Kuroso, N. Ohyabu, W. Wang, S. Fujii and I. Kitagawa, Chem. Pharm. Bull., 1994, 42, 2196 Search PubMed.
- M. Eggen, C. J. Mossman, S. B. Buck, S. K. Nair, L. Bhat, S. M. Ali, E. A. Reiff, T. C. Boge and G. I. Georg, J. Org. Chem., 2000, 65, 7792 CrossRef CAS.
- G. R. Pettit, Z. A. Cichacz, M. D. Williams, A.-C. Dorsaz, D. C. Brune, M. R. Boyd and R. L. Cerny, Bioorg. Med. Chem. Lett., 1994, 4, 2091 CrossRef CAS.
- G. R. Pettit, B. E. Toki, J.-P. Xu and D. C. Brune, J. Nat. Prod., 2000, 63, 22 CrossRef CAS.
- A. Casapullo, E. Finemore, L. Minale and F. Zollo, Tetrahedron Lett., 1993, 34, 6297 CrossRef CAS.
- B. B. Snider, F. Song and B. M. Foxman, J. Org. Chem., 2000, 65, 793 CrossRef CAS.
- A. Rudi, M. Schleyer and Y. Kashmann, J. Nat. Prod., 2000, 63, 1434 CrossRef CAS.
- U. Venkatesham, M. R. Rao and Y. Venkateswarlu, J. Nat. Prod., 2000, 63, 1318 CrossRef CAS.
- D. C. Dunbar, J. M. Rimoldi, A. M. Clark, M. Kelly and M. T. Hamann, Tetrahedron, 2000, 56, 8795 CrossRef CAS.
- S. Carmely and Y. Kashman, Tetrahedron Lett., 1987, 28, 3003 CrossRef CAS.
- S. Ohta, N. Tsuno, S. Nakamura, N. Taguchi, M. Yamashita, I. Kawasaki and M. Fujieda, Heterocycles, 2000, 53, 1939 Search PubMed.
- P. Ciminiello, E. Fattorusso, S. Magno and A. Mangoni, Tetrahedron, 1989, 45, 3873 CrossRef CAS.
- S. Ohta, N. Tsuno, K. Maeda, S. Nakamura, N. Taguchi, M. Yamashita and I. Kawasaki, Tetrahedron Lett., 2000, 41, 4623 CrossRef CAS.
- B. Moon, Y. C. Park, J. B. McClintock and B. J. Baker, Tetrahedron, 2000, 56, 9057 CrossRef CAS.
- J. M. Frincke and D. J. Faulkner, J. Am. Chem. Soc., 1982, 104, 265 CrossRef CAS.
- M. Kobayashi, S. R. Rao, R. Chavakula and N. S. Sarma, J. Chem. Res. (S), 1994, 282 Search PubMed.
- R. A. Edrada, P. Proksch , V. Wray, R. Christ, L. Witte and R. W. M. Van Soest, J. Nat. Prod., 1996, 59, 973 CrossRef CAS.
- B. Kesteleyn and N. De Kimpe, J. Org. Chem., 2000, 65, 635 CrossRef CAS.
- S. A. Fedoreev, N. G. Prokof'eva, V. A. Denisenko and N. M. Rebuchuk, Khim-Farm. Zh., 1988, 22, 943 Search PubMed.
- Y. Kashman, A. Rudi, S. Hirsch, S. Isaacs, D. Green, D. Blasberger and S. Carmely, New J. Chem., 1990, 14, 729 Search PubMed.
- A. J. Walz and R. J. Sundberg, J. Org. Chem., 2000, 65, 8001 CrossRef CAS.
- G. W. Chan, T. Francis, D. R. Thureen, P. H. Offen, N. J. Pierce, J. W. Westley, R. K. Johnson and D. J. Faulkner, J. Org. Chem., 1993, 58, 2544 CrossRef CAS.
- C. Peschko and W. Steglich, Tetrahedron Lett., 2000, 41, 9477 CrossRef CAS.
- A. R. Carroll and P. J. Scheuer, Tetrahedron, 1990, 46, 6637 CrossRef CAS.
- G. M. Nicholas and T. F. Molinski, Tetrahedron, 2000, 56, 2921 CrossRef CAS.
- S. Tsukamoto, M. Takahashi, S. Matsunaga, N. Fusetani and R. W. M. van Soest, J. Nat. Prod., 2000, 63, 682 CrossRef CAS.
- K. Hirano, T. Kubota, M. Tsuda, Y. Mikami and J. Kobayashi, Chem. Pharm. Bull., 2000, 48, 974 Search PubMed.
- T. Iwagawa, M. Kaneko, H. Okamura, M. Nakatani, R. W. M. van Soest and M. Shiro, J. Nat. Prod., 2000, 63, 1310 CrossRef CAS.
- J. I. Jiménez, G. Goetz, C. M. S. Mau, W. Y. Yoshida, P. J. Scheuer, R. T. Williamson and M. Kelly, J. Org. Chem., 2000, 65, 8465 CrossRef CAS.
- B.-N. Zhou, C. Slebodnick, R. K. Johnson, M. R. Mattern and D. G. I. Kingston, Tetrahedron, 2000, 56, 5781 CrossRef CAS.
- R. Sakai, S. Kohmoto, T. Higa, C. W. Jefford and G. Bernardinelli, Tetrahedron Lett., 1987, 28, 5493 CrossRef CAS.
- D. I. MaGee and E. J. Beck, Can. J. Chem., 2000, 78, 1060 CrossRef CAS.
- Y. R. Torres, R. G. S. Berlinck, A. Magalhães, A. B. Schefer, A. G. Ferreira, E. Hajdu and G. Muricy, J. Nat. Prod., 2000, 63, 1098 CrossRef CAS.
- D. E. Williams, P. Lassota and R. J. Andersen, J. Org. Chem., 1998, 63, 4838 CrossRef CAS.
- A. Fürstner and A. Rumbo, J. Org. Chem., 2000, 65, 2608 CrossRef CAS.
- E. Fattorusso and O. Taglialatela-Scafati, Tetrahedron Lett., 2000, 41, 9917 CrossRef CAS.
- N. S. Reddy and Y. Venkateswarlu, Biochem. Syst. Ecol., 2000, 28, 1035 CrossRef CAS.
- A. Umeyama, S. Ito, E. Yasuda, S. Arihara and T. Yamada, J. Nat. Prod., 1998, 61, 1433 CrossRef CAS.
- E. E. Garcia, L. E. Benjamin and R. I. Fryer, J. Chem. Soc., Chem. Commun., 1973, 78 RSC.
- T. Lindel and M. Hochgürtel, J. Org. Chem., 2000, 65, 2806 CrossRef CAS.
- J. Kobayashi, F. Kanda, M. Ishibashi and H. Shigemori, J. Org. Chem., 1991, 56, 4574–4576 CrossRef CAS.
- K. Namba, T. Shinada, T. Teramoto and Y. Ohfune, J. Am. Chem. Soc., 2000, 122, 10708 CrossRef CAS.
- G. M. Sharma, J. S. Buyer and M. W. Pomerantz, J. Chem. Soc., Chem. Commun., 1980, 435 RSC.
- A. Supriyono, B. Schwartz, V. Wray, L. Witte, W. E. G. Müller, R. van Soest, W. Sumaryono and P. Proksch, Z. Naturforsch., C: Biosci., 1995, 50, 669 Search PubMed.
- A. C. B. Sosa, K. Yakushijin and D. A. Horne, J. Org. Chem., 2000, 65, 610 CrossRef CAS.
- M. Tsuda, H. Uemoto and J. Kobayashi, Tetrahedron Lett., 1999, 40, 5709 CrossRef CAS.
- A. C. B. Sosa, K. Yakushijin and D. A. Horne, Org. Lett., 2000, 2, 3443 CrossRef CAS.
- I. Carlett, B. Banaigs and P. Amade, J. Nat. Prod., 2000, 63, 981 CrossRef CAS.
- I. Erdoğan, B. Şener and T. Higa, Biochem. Syst. Ecol., 2000, 28, 793 CrossRef CAS.
- H. R. Bokesch, L. K. Pannell, T. C. McKee and M. R. Boyd, Tetrahedron Lett., 2000, 41, 6305 CrossRef CAS.
- A. Casapullo, G. Bifulco, I. Bruno and R. Riccio, J. Nat. Prod., 2000, 63, 447 CrossRef CAS.
- A. Cutignano, G. Bifulco, I. Bruno, A. Casapullo, L. Gomez-Paloma and R. Riccio, Tetrahedron, 2000, 56, 3743 CrossRef CAS.
- K. Bartik, J.-C. Braekman, D. Daloze, C. Stoller, J. Huysecom, G. Vandevyver and R. Ottinger, Can. J. Chem., 1987, 65, 2118 CAS.
- S. Sakemi and H. H. Sun, J. Org. Chem., 1991, 56, 4304 CrossRef CAS.
- I. Mancini, G. Guella, C. Debitus, J. Waikedre and F. Pietra, Helv. Chim. Acta, 1996, 79, 2075 CrossRef CAS.
- F. Y. Miyake, K. Yakushijin and D. A. Horne, Org. Lett., 2000, 2, 2121 CrossRef CAS.
- S. A. Morris and R. J. Andersen, Tetrahedron, 1990, 46, 715 CrossRef CAS.
- T. Kawasaki, H. Enoki, K. Matsumura, M. Ohyama, M. Inagawa and M. Sakamoto, Org. Lett., 2000, 2, 3027 CrossRef CAS.
- F. Y. Miyake, K. Yakushijin and D. A. Horne, Org. Lett., 2000, 2, 3185 CrossRef CAS.
- J. Ford and R. J. Capon, J. Nat. Prod., 2000, 63, 1527 CrossRef CAS.
- G. R. Pettit, J. C. Knight, J. C. Collins, D. L. Herald, R. K. Pettit, M. R. Boyd and V. G. Young, J. Nat. Prod., 2000, 63, 793 CrossRef CAS.
- J. C. Braekman, D. Daloze, R. Tavares, E. Hadju and R. W. M. Van Soest, J. Nat. Prod., 2000, 63, 193 CrossRef CAS.
- I. Ohtani, T. Kusumi, H. Kakisawa, Y. Kashman and S. Hirsch, J. Am. Chem. Soc., 1992, 114, 8472 CrossRef CAS.
- R. Tavares, D. Daloze, J. C. Braekman, E. Hajdu, G. Muricy and R. W. M. Van Soest, Biochem. Syst. Ecol., 1994, 22, 645 CrossRef CAS.
- R. G. S. Berlinck, J. C. Braekman, D. Daloze, I. Bruno, R. Riccio, S. Ferri, S. Spampinato and E. Speroni, J. Nat. Prod., 1993, 56, 1007 CrossRef CAS.
- Y. Venkateswarlu, M. V. R. Reddy, P. R. Rao and J. V. Rao, Indian J. Chem., Sect. B Org. Chem. Incl. Med. Chem., 1999, 38, 254 Search PubMed.
- D. S. Coffey, A. I. McDonald, L. E. Overman, M. H. Rabinowitz and P. A. Renhowe, J. Am. Chem. Soc., 2000, 122, 4893 CrossRef CAS.
- E. A. Jares-Erijman, A. L. Ingrum, J. R. Carney, K. L. Rinehart and R Sakai, J. Org. Chem., 1993, 58, 4805 CrossRef CAS.
- D. S. Coffey, L. E. Overman and F. Stappenbeck, J. Am. Chem. Soc., 2000, 122, 4904 CrossRef CAS.
- R. J. Capon, F. Rooney and L. M. Murray, J. Nat. Prod., 2000, 63, 261 CrossRef CAS.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso, S. Magno and M. Pansini, J. Nat. Prod., 2000, 63, 263 CrossRef CAS.
- C. Lacy and P. J. Scheuer, J. Nat. Prod., 2000, 63, 119 CrossRef CAS.
- S. A. Ross, J. D. Weete, R. F. Schinazi, S. S. Wirtz, P. Tharnish, P. J. Scheuer and M. T. Hamann, J. Nat. Prod., 2000, 63, 501 CrossRef CAS.
- K. Hirano, T. Kubota, M. Tsuda, K. Watanabe, J. Fromont and J. Kobayashi, Tetrahedron, 2000, 56, 8107 CrossRef CAS.
- Y. Okamoto, M. Ojika, S. Kato and Y. Sakagami, Tetrahedron, 2000, 56, 5813 CrossRef CAS.
- R. D. Encarnación, E. Dandoval, J. Malmstrøm and C. Christophersen, J. Nat. Prod., 2000, 63, 874 CrossRef.
- J. Shin, H.-S. Lee, Y. Seo, J.-R. Rho, K. W. Cho and V. J. Paul, Tetrahedron, 2000, 56, 9071 CrossRef CAS.
- N. B. Pham, M. S. Butler and R. J. Quinn, J. Nat. Prod., 2000, 63, 393 CrossRef CAS.
- B. F. Bowden, L. Towerzey and P. C. Junk, Aust. J. Chem., 2000, 53, 299 CrossRef CAS.
- G. M. Cameron, B. L. Stapleton, S. M. Simonsen, D. J. Brecknell and M. J. Garson, Tetrahedron, 2000, 56, 5247 CrossRef CAS.
- M. Salmoun, C. Devijver, D. Daloze, J. C. Braekman, R. Gomez, M. de Kluijver and R. W. M. Van Soest, J. Nat. Prod., 2000, 63, 452 CrossRef CAS.
- E. Goclik, G. M. König, A. D. Wright and R. Kaminsky, J. Nat. Prod., 2000, 63, 1150 CrossRef CAS.
- J. H. Kwak, F. J. Schmitz and M. Kelly, J. Nat. Prod., 2000, 63, 1153 CrossRef CAS.
- C. Giannini, C. Debitus, I. Posadas, M. Payá and M. V. D'Auria, Tetrahedron Lett., 2000, 41, 3257 CrossRef CAS.
- A. D. Patil, A. J. Freyer, L. Killmer, P. Offen, B. Carte, A. J. Jurewicz and R. K. Johnson, Tetrahedron, 1997, 53, 5047 CrossRef CAS.
- M. Inoue, A. J. Frontier and S. J. Danishefsky, Angew. Chem., Int. Ed., 2000, 39, 761 CrossRef CAS.
- A. Casapullo, L. Minale and F. Zollo, J. Nat. Prod., 1993, 56, 527 CrossRef CAS.
- A. F. Barrero, E. J. Alvarez-Manzaneda, R. Chabhoun and C. G. Díaz, Synlett, 2000, 1561 CAS.
- Y.-C. Shen, M.-C. Hung, C. V. S. Prakash and J.-J. Wang, J. Chin. Chem. Soc., 2000, 47, 567 Search PubMed.
- D. Tasdemir, G. P. Concepción, G. C. Mangalindan, M. K. Harper, E. Hajdu and C. M. Ireland, Tetrahedron, 2000, 56, 9025 CrossRef CAS.
- D. Tasdemir, G. P. Concepción, G. C. Mangalindan, M. K. Harper, E. Hajdu and C. M. Ireland, Tetrahedron, 2001, 57, 5681 CrossRef CAS.
- C. L. Blackburn and D. J. Faulkner, Tetrahedron, 2000, 56, 8429 CrossRef CAS.
- P. Crews and B. Harrison, Tetrahedron, 2000, 56, 9036 CrossRef.
- R. J. Capon, M. Miller and F. Rooney, J. Nat. Prod., 2000, 63, 821 CrossRef CAS.
- K. L. McPhail, D. E. A. Rivett, D. E. Lack and M. T. Davies-Coleman, Tetrahedron, 2000, 56, 9391 CrossRef CAS.
- N. S. Reddy, U. Venkatesham, T. P. Rao and Y. Venkateswarlu, Indian J. Chem., Sect. B Org. Chem. Incl. Med. Chem., 2000, 39, 393 Search PubMed.
- R. J. Clark, B. L. Stapleton and M. J. Garson, Tetrahedron, 2000, 56, 3071 CrossRef CAS.
- A. Y. Pham, T. Ichiba, W. Y. Yoshida, P. J. Scheuer, T. Uchida, J. Tanaka and T. Higa, Tetrahedron Lett., 1991, 32, 4843 CrossRef CAS.
- H. He, J. Salvá, R. F. Catalos and D. J. Faulkner, J. Org. Chem., 1992, 57, 3191 CrossRef CAS.
- A. Srikrishna and S. J. Gharpure, J. Chem. Soc., Perkin Trans. 1, 2000, 3191 RSC.
- H. Hirota, S. Matsunaga and N. Fusetani, Tetrahedron Lett., 1990, 31, 4163 CrossRef CAS.
- G. A. Whitlock and E. M. Carreira, Helv. Chim. Acta, 2000, 83, 2007 CrossRef CAS.
- D. Vuong and R. J. Capon, J. Nat. Prod., 2000, 63, 1684 CrossRef CAS.
- M. Hyosu and J. Kimura, J. Nat. Prod., 2000, 63, 422 CrossRef CAS.
- S. S. Mitchell, M. K. Harper and D. J. Faulkner, Tetrahedron, 1999, 55, 10887 CrossRef CAS.
- N. Shoji, A. Umeyama, M. Teranaka and S. Arihara, J. Nat. Prod., 1996, 59, 448 CrossRef CAS.
- J. Bonjoch, J. Cuesta, S. Díaz and A. González, Tetrahedron Lett., 2000, 41, 5669 CrossRef CAS.
- R. C. Cambie, A. R. Lal, M. R. Kernan and P. R. Bergquist, J. Nat. Prod., 1995, 58, 940 CrossRef CAS.
- K. D. Wellington, R. C. Cambie, P. S. Rutledge and P. R. Bergquist, J. Nat. Prod., 2000, 63, 79 CrossRef CAS.
- T. Yosief, A. Rudi and Y. Kashman, J. Nat. Prod., 2000, 63, 299 CrossRef CAS.
- Y. W. Guo and E. Trivellone, Chin. Chem. Lett., 2000, 11, 327 Search PubMed.
- M. S. Butler and R. J. Capon, Aust. J. Chem., 1992, 45, 1705 CrossRef CAS.
- A. F. Barrero, E. J. Alvarez-Manzaneda, R. Chahboun, J. M. Cuerva and A. Segovia, Synlett, 2000, 1269 CAS.
- G. Kirsch, G. M. König, A. D. Wright and R. Kaminsky, J. Nat. Prod., 2000, 63, 825 CrossRef CAS.
- A. Soriente, A. Crispino, M. De Rosa, S. De Rosa, A. Scettri, G. Scognamiglio, R. Villano and G. Sodano, Eur. J. Org. Chem., 2000, 947 CrossRef CAS.
- K. Iguchi, Y. Shimada and Y. Yamada, J. Org. Chem., 1992, 57, 522 CrossRef CAS.
- P. Basabe, A. Diego, D. Díez, I. S. Marcos and J. G. Urones, Synlett, 2000, 1807 CAS.
- H. Miyaoka, S. Nishijima, H. Mitome and Y. Yamada, J. Nat. Prod., 2000, 63, 1369 CrossRef CAS.
- S. De Marino, M. Iorizzi, F. Zollo, C. Debitus, J.-L. Menou, L. F. Ospina, M. J. Alcaraz and M. Payá, J. Nat. Prod., 2000, 63, 322 CrossRef CAS.
- J. I. Jiménez, W. Y. Yoshida, P. J. Scheuer, E. Lobkovsky, J. Clardy and M. Kelly, J. Org. Chem., 2000, 65, 6837 CrossRef CAS.
- J. I. Jiménez, W. Y. Yoshida, P. J. Scheuer and M. Kelly, J. Nat. Prod., 2000, 63, 1388 CrossRef CAS.
- S. P. Gunasekera, P. J. McCarthy, M. Kelly-Borges, E. Lobkovsky and J. Clardy, J. Am. Chem. Soc., 1996, 118, 8759 CrossRef CAS.
- H. Miyaoka, Y. Kajiwara and Y. Yamada, Tetrahedron Lett., 2000, 41, 911 CrossRef CAS.
- M. Takahashi, K. Dodo, Y. Hashimoto and R. Shirai, Tetrahedron Lett., 2000, 41, 2111 CrossRef CAS.
- E. Piers, S. Caillé and G. Chen, Org. Lett., 2000, 2, 2483 CrossRef CAS.
- D. Demeke and C. J. Forsyth, Org. Lett., 2000, 2, 3177 CrossRef CAS.
- A. Qureshi and D. J. Faulkner, J. Nat. Prod., 2000, 63, 841 CrossRef CAS.
- S. H. Xu and L. M. Zeng, Chin. Chem. Lett., 2000, 11, 531 Search PubMed.
- A. Umeyama, S. Ito, A. Yoshigaki and S. Arihara, J. Nat. Prod., 2000, 63, 1540 CrossRef CAS.
- A. Gauvin, J. Smadja, M. Ankin, R. Faure and E.-M. Gaydou, Can. J. Chem., 2000, 78, 986 CrossRef CAS.
- P. de A. Leone, J. Redburn, J. N. A. Hooper and R. J. Quinn, J. Nat. Prod., 2000, 63, 694 CrossRef CAS.
- V. Costantino, C. Dell'Aversano, E. Fattorusso and A. Mangoni, Steroids, 2000, 65, 138 CrossRef CAS.
- A. Umeyama, K. Adachi, S. Ito and S. Arihara, J. Nat. Prod., 2000, 63, 1175 CrossRef CAS.
- P. Stead, S. Hiscox, P. S. Robinson, N. B. Pike, P. J. Sidebottom, A. D. Roberts, N. L. Taylor, A. E. Wright, S. A. Pomponi and D. Langley, Bioorg. Med. Chem. Lett., 2000, 10, 661 CrossRef CAS.
- L. N. Li, H.-T. Li, R. W. Lang, T. Itoh, D. Sica and C. Djerassi, J. Am. Chem. Soc., 1982, 104, 6726 CrossRef CAS.
- G. A. Doss and C. Djerassi, J. Am. Chem. Soc., 1988, 110, 8124 CrossRef CAS.
- A. Kurek-Tyrlik, K. Minksztym and J. Wicha, Eur. J. Org. Chem., 2000, 1027 CrossRef CAS.
- J. Rodríguez, L. Nuñez, S. Peixinho and C. Jiménez, Tetrahedron Lett., 1997, 38, 1833 CrossRef CAS.
- N. B. Kovganko and Y. G. Chernov, Chem. Nat. Compd., 2000, 36, 189 Search PubMed.
- A. Zampella, M. V. D'Auria, C. Debitus and J.-L. Menou, J. Nat. Prod., 2000, 63, 943 CrossRef CAS.
- N. Oku, S. Matsunaga, S. Wada, S. Watabe and N. Fusetani, J. Nat. Prod., 2000, 63, 205 CrossRef CAS.
- M.-L. Bourguet-Kondracki, A. Longeon, C. Debitus and M. Guyot, Tetrahedron Lett., 2000, 41, 3087 CrossRef CAS.
- H.-S. Lee, Y. Seo, K. W. Cho, J.-R. Rho, J. Shin and V. J. Paul, J. Nat. Prod., 2000, 63, 915 CrossRef CAS.
- M. Shatz, T. Yosief and Y. Kashman, J. Nat. Prod., 2000, 63, 1554 CrossRef CAS.
- V. Costantino, E. Fattorusso, C. Imperatore and A. Mangoni, Tetrahedron, 2000, 56, 3781 CrossRef CAS.
- V. Costantino, E. Fattorusso, A. Mangoni, M. Di Rosa and A. Ianaro, Tetrahedron, 2000, 56, 1393 CrossRef CAS.
- F. S. De Guzman and F. J. Schmitz, J. Org. Chem., 1991, 56, 55 CrossRef.
- D. Nozawa, H. Takikawa and K. Mori, J. Chem. Soc., Perkin Trans. 1, 2000, 2043 RSC.
- V. Anjaneyulu, P. V. Subba Rao, P. Radhika, H. Laatsch and L. N. Misra, Indian J. Chem., Sect. B Org. Chem. Incl. Med. Chem., 2000, 39, 121 Search PubMed.
- M. Y. Wang, Y. Luo and J. Y. Su, Chin. Chem. Lett., 2000, 11, 783 Search PubMed.
- A. S. R. Anjaneyulu, M. V. R. Krishna Murthy, P. M. Gowri, M. J. R. V. Venugopal and H. Laatsch, J. Nat. Prod., 2000, 63, 1425 CrossRef CAS.
- A. Agalias, N. Mihopoulos, M. Tsoukatou, L. Marinos, C. Vagias, C. Harvala and V. Roussis, Z. Naturforsh., C: Biosci., 2000, 55, 425 Search PubMed.
- B. H. Bae, K. S. Im, W. C. Choi, J. Hong, C.-O. Lee, J. S. Choi, B. W. Son, J.-I. Song and J. H. Jung, J. Nat. Prod., 2000, 63, 1511 CrossRef CAS.
- N. Lindquist, N. Shigematsu and L. Pannell, J. Nat. Prod., 2000, 63, 1290 CrossRef CAS.
- J.-H. Sheu, K.-C. Hung, G.-H. Wang and C.-Y. Duh, J. Nat. Prod., 2000, 63, 1603 CrossRef CAS.
- M. Yasumoto, K. Mada, T. Ooi and T. Kusumi, J. Nat. Prod., 2000, 63, 1534 CrossRef CAS.
- J. A. Palermo, M. F. R. Brasco, C. Spagnuolo and A. M. Seldes, J. Org. Chem., 2000, 65, 4482 CrossRef CAS.
- R. A. Edrada, V. Wray, L. Witte, L. van Ofwegen and P. Proksch, Z. Naturforsch., C: Biosci, 2000, 55, 82 Search PubMed.
- P. Ramesh and Y. Venkateswarlu, J. Chem. Res. (S), 2000, 48–50 Search PubMed.
- M. R. Rao, K. V. Sridevi, U. Venkatesham, T. P. Rao, S. S. Lee and Y. Venkareswarlu, J. Chem. Res. (S), 2000, 245 Search PubMed.
- A. S. R. Anjaneyulu and M. V. R. Krishna Murthy, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2000, 39, 42 Search PubMed.
- A. S. R. Anjaneyulu and P. M. Gowri, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2000, 39, 773 Search PubMed.
- H. T. D'Armas, B. S. Mootoo and W. R. Reynolds, J. Nat. Prod., 2000, 63, 1593 CrossRef CAS.
- F. J. McEnroe and W. Fenical, Tetrahedron, 1978, 34, 1661 CrossRef CAS.
- C. Fuganti and S. Serra, J. Chem. Soc., Perkin Trans. 1, 2000, 3758 RSC.
- H. R. Bokesch, T. C. McKee, J. H. Cardellina II and M. R. Boyd, Tetrahedron Lett., 1996, 37, 3259 CrossRef CAS.
- H.-Y. Lee and B. G. Kim, Org. Lett., 2000, 2, 1951 CrossRef CAS.
- Y. Kashman, S. Carmely and A. Groweiss, J. Org. Chem., 1981, 46, 3592 CrossRef CAS.
- C. A. Gray, M. T. Davies-Coleman and M. H. Schleyer, J. Nat. Prod., 2000, 63, 1551 CrossRef CAS.
- C.-Y. Duh, S.-K. Wang and Y.-L. Weng, Tetrahedron Lett., 2000, 41, 1401 CrossRef CAS.
- C.-Y. Duh, S.-K. Wang, B.-T. Huang and C.-F. Dai, J. Nat. Prod., 2000, 63, 884 CrossRef CAS.
- M. A. Rashid, K. R. Gustafson and M. R. Boyd, J. Nat. Prod., 2000, 63, 531 CrossRef CAS.
- C.-Y. Duh, S.-K. Wang, S.-G. Chung, G.-C. Chou and C.-F. Dai, J. Nat. Prod., 2000, 63, 1634 CrossRef CAS.
- M. Iwashima, Y. Matsumoto, H. Takahashi and K. Iguchi, J. Nat. Prod., 2000, 63, 1647 CrossRef CAS.
- J. Su, R. Yang, Y. Kuang and L. Zeng, J. Nat. Prod., 2000, 63, 1543 CrossRef CAS.
- R.-L. Yang, L.-M. Zeng, J.-Y. Su and H. Li, Acta Chim. Sin. (Engl. Ed.), 2000, 10, 1186 Search PubMed.
- A. D. Rodríguez, I. C. Piña, J. J. Soto, D. R. Rojas and C. L. Barnes, Can. J. Chem., 1995, 73, 643 CAS.
- A. D. Rodríguez, J. J. Soto and I. C. Piña, J. Nat. Prod., 1995, 58, 1209 CrossRef CAS.
- A. D. Rodríguez, J. J. Soto and C. L. Barnes, J. Org. Chem., 2000, 65, 7700 CrossRef CAS.
- L. Xu, B. O. Patrick, M. Roberge, T. Allen, L. van Ofwegen and R. J. Andersen, Tetrahedron, 2000, 56, 9031 CrossRef.
- V. Anjaneyulu, T. N. Makarieva, S. G. Ilyin, A. S. Dmitrenok, P. Radhika, P. V. Subbarao, V. V. Nesterov, M. Y. Antipin and V. A. Stonik, J. Nat. Prod., 2000, 63, 109 CrossRef.
- M.-C. Chai, S.-K. Wang, C.-F. Dai and C.-Y. Duh, J. Nat. Prod., 2000, 63, 843 CrossRef CAS.
- B. F. Bowden, J. C. Coll and D. M. Tapiolas, Aust. J. Chem., 1983, 36, 2289 CAS.
- J. Lan, Z. Liu, H. Yuan, L. Peng, W.-D. Z. Li, Y. Li, Y. Li and A. S. C. Chen, Tetrahedron Lett., 2000, 41, 2181 CrossRef CAS.
- B. F. Bowden, J. C. Coll, W. Hicks, R. Kazlauskas and S. J. Mitchell, Aust. J. Chem., 1978, 31, 2707 CAS.
- B. F. Bowden, J. C. Coll and S. J. Mitchell, Aust. J. Chem., 1980, 33, 879 CAS.
- Y. Li, Z. Liu, J. Lan, J. Li, L. Peng, W. Z. Li, Y. Li and A. S. C. Chan, Tetrahedron Lett., 2000, 41, 7465 CrossRef CAS.
- Z. Liu, W. Z. Li, L. Peng, Y. Li and Y. Li, J. Chem. Soc., Perkin Trans. 1, 2000, 4250 RSC.
- A. D. Rodríguez and C. Ramírez, Org. Lett., 2000, 2, 507 CrossRef CAS.
- A. D. Rodríguez, C. Ramírez and Y.-P. Shi, J. Org. Chem., 2000, 65, 6682 CrossRef CAS.
- A. D. Rodríguez, C. Ramírez, I. I. Rodríguez and C. L. Barnes, J. Org. Chem., 2000, 65, 1390 CrossRef CAS.
- A. D. Rodríguez, C. Ramírez, V. Medina and Y.-P. Shi, Tetrahedron Lett., 2000, 41, 5177 CrossRef CAS.
- A. D. Rodríguez and Y.-P. Shi, Tetrahedron, 2000, 56, 9015 CrossRef CAS.
- A. Ata and R. G. Kerr, Tetrahedron Lett., 2000, 41, 5821 CrossRef CAS.
- A. C. Coleman and R. G. Kerr, Tetrahedron, 2000, 56, 9569 CrossRef CAS.
- V. Roussis, Z. Wu, W. Fenical, S. A. Strobel, G. D. Van Duyne and J. Clardy, J. Org. Chem., 1990, 55, 4916 CrossRef CAS.
- J. Tanaka, N. Ogawa, J. Liang, T. Higa and D. G. Gravalos, Tetrahedron, 1993, 49, 811 CrossRef CAS.
- S. E. Lazerwith, T. W. Johnson and E. J. Corey, Org. Lett., 2000, 2, 2389 CrossRef CAS.
- A. Ata and R. J. Kerr, Heterocycles, 2000, 53, 717 Search PubMed.
- A. D. Rodríguez, J.-C. Shi and Y.-P. Shi, J. Org. Chem., 2000, 65, 3192 CrossRef CAS.
- A. D. Rodríguez and Y.-P. Shi, J. Nat. Prod., 2000, 63, 1548 CrossRef CAS.
- A. D. Rodríguez and Y.-P. Shi, J. Org. Chem., 2000, 65, 5839 CrossRef CAS.
- R. de A. Epifanio, L. M. Maia and W. Fenical, J. Braz. Chem. Soc., 2000, 11, 584 Search PubMed.
- H. Miyaoka, H. Mitome, M. Nakano and Y. Yamada, Tetrahedron, 2000, 56, 7737 CrossRef CAS.
- T. Iwagawa, K. Nakamura, T. Hirose, H. Okamura and M Nakatani, J. Nat. Prod., 2000, 63, 468 CrossRef CAS.
- J.-R. Rho, H.-S. Lee, Y. Seo, K. W. Cho and J. Shin, J. Nat. Prod., 2000, 63, 254 [erratum J. Nat. Prod., 2000, 63, 1051] Search PubMed.
- R. E. Schwartz and P. J. Scheuer, Tetrahedron, 1981, 37, 2725 CrossRef CAS.
- D. Renneberg, H. Pfander and C. J. Leumann, J. Org. Chem., 2000, 65, 9069 CrossRef CAS.
- Y. Seo, K. W. Cho, S. Chung and J. Shin, Nat. Prod. Lett., 2000, 14, 197–205 Search PubMed.
- P. Sharma and M. Alam, J. Chem. Soc., Perkin Trans. 1, 1988, 2537 RSC.
- D. Friedrich, R. W. Doskotch and L. A. Paquette, Org. Lett., 2000, 2, 1879 CrossRef CAS.
- L. A. Paquette, O. M. Moradei, P. Bernardelli and T. Lange, Org. Lett., 2000, 2, 1875 CrossRef CAS.
- L. E. Overman and L. D. Pennington, Org. Lett., 2000, 2, 2683 CrossRef CAS.
- I. Mancini, G. Guella, H. Zibrowius and F. Pietra, Helv. Chim. Acta, 2000, 83, 1561 CrossRef CAS.
- N. B. Pham, M. S. Butler, P. C. Healy and R. J. Quinn, J. Nat. Prod., 2000, 63, 318 CrossRef CAS.
- C. Subrahmanyam, S. Ratnakumar and R. S. Ward, Tetrahedron, 2000, 56, 4585 CrossRef CAS.
- T. Iwagawa, T. Hirose, K. Takayama, H. Okamura, M. Nakatani, M. Doe and K. Takemura, Heterocycles, 2000, 53, 1789 Search PubMed.
- P.-J. Sung, S.-L. Wu, H.-J. Fang, M. Y. Chang, J.-Y. Wu, L.-S. Fang and J.-H. Sheu, J. Nat. Prod., 2000, 63, 1483 CrossRef CAS.
- T. Lindel, P. R. Jensen, W. Fenical, B. H. Long, A. M. Casazza, J. Carbonari and C. R. Fairchild, J. Am. Chem. Soc., 1997, 119, 8744 CrossRef CAS.
- B. Cinel, M. Roberge, H. Behrisch, L. van Ofwegen, C. B. Castro and R. J. Andersen, Org. Lett., 2000, 2, 257 CrossRef CAS.
- A. S. R. Anjaneyulu, M. V. R. Krishna Murthy and P. M. Gowri, J. Nat. Prod., 2000, 63, 112 CrossRef CAS.
- J.-R. Rho, H.-S. Lee, Y. Seo, K. W. Cho and J. Shin, Bull. Korean Chem. Soc., 2000, 21, 518 Search PubMed.
- M. Kobayashi and F. Kanda, J. Chem. Soc., Perkin Trans. 1, 1991, 1177 RSC.
- P. Ramesh, V. L. N. Reddy, N. S. Reddy and Y. Venkateswarlu, J. Nat. Prod., 2000, 63, 1420 CrossRef CAS.
- L. Garrido, E. Zubía, M. J. Ortega and J. Salvá, Steroids, 2000, 65, 85 CrossRef CAS.
- H. Dong, Y.-L. Gou, R. M. Kini, H.-X. Xu, S.-X. Chen, S. L. M. Teo and P. P.-H. But, Chem. Pharm. Bull., 2000, 48, 1087 Search PubMed.
- J.-H. Sheu, S.-P. Chen, P.-J. Sung, M. Y. Chiang and C.-F. Dai, Tetrahedron Lett., 2000, 41, 7885 CrossRef CAS.
- C. Subrahmanyam and S. R. Kumar, J. Chem. Res. (S), 2000, 182 Search PubMed.
- M. Iwashima, K. Nara and K. Iguchi, Steroids, 2000, 65, 130 CrossRef CAS.
- K. Yoshikawa, S. Kanekuni, M. Hanahusa, S. Arihara and T. Ohta, J. Nat. Prod., 2000, 63, 670 CrossRef CAS.
- H. T. D'Armas, B. S. Mootoo and W. F. Reynolds, J. Nat. Prod., 2000, 63, 1669 CrossRef CAS.
- J.-H. Sheu, K.-C. Chang and C.-Y. Duh, J. Nat. Prod., 2000, 63, 149 CrossRef CAS.
- S. Naz, R. G. Kerr and R. Narayanan, Tetrahedron Lett., 2000, 41, 6035 CrossRef CAS.
- L. F. Maia, R. de A. Epifanio and W. Fenical, J. Nat. Prod., 2000, 63, 1427 CrossRef CAS.
- T. Shindo, A. Sato, N. Kasanuki, K. Hasegawa, S. Sato, T. Iwata and T. Hata, Experientia, 1993, 49, 177 Search PubMed.
- T. R. Kelly, Y. Fu, J. T. Sieglen, Jr. and H. De Silva, Org. Lett., 2000, 2, 2351–2352 CrossRef CAS.
- H.-P. Zhang, Y. Kamono, H. Kizu, H. Itokawa, G. R. Pettit and C. Herald, Chem. Lett., 1994, 2271 CAS.
- Y. Kamono, A. Kotake, H. Hashima, I. Hayakawa, H. Hiraide, H.-P. Zhang, H. Kizu, K. Komiyama, M. Hayashi and G. R. Pettit, Collect. Czech. Chem. Commun., 1999, 64, 1147 CrossRef CAS.
- H. Hashima, M. Hayashi, Y. Kamano and N. Sato, Bioorg. Med. Chem., 2000, 8, 1757 CrossRef CAS.
- D. E. Schaufelberger, G. N. Chmurny, J. A. Beutler, M. P. Koleck, A. B. Alvarado, B. W. Schaufelberger and G. M. Muschik, J. Org. Chem., 1991, 56, 2895 CrossRef CAS.
- K. Ohmori, Y. Ogawa, T. Obitsu, Y. Ishikawa, S. Nishiyama and S. Yamamura, Angew. Chem., Int. Ed., 2000, 29, 2290 CrossRef CAS.
- W. A. Gallimore, D. L. Galario, C. Lacy, Y. Zhu and P. J. Scheuer, J. Nat. Prod., 2000, 63, 1022 CrossRef CAS.
- W. A. Gallimore and P. J. Scheuer, J. Nat. Prod., 2000, 63, 1422 CrossRef CAS.
- S. N. Federov, L. K. Shubina, A. I. Kalinovsky, E. G. Lyakhova and V. A. Stonik, Tetrahedron Lett., 2000, 41, 1979 CrossRef CAS.
- M. Wessels, G. M. König and A. D. Wright, J. Nat. Prod., 2000, 63, 920 CrossRef CAS.
- M. T. Hamann, C. S. Otto, P. J. Scheuer and D. C. Dunbar, J. Org. Chem., 1996, 61, 6594 CrossRef CAS.
- À. López-Macià, J. C. Jiménez, M. Royo, E. Giralt and F. Albericio, Tetrahedron Lett., 2000, 41, 9765 CrossRef CAS.
- M. Gavagnin, E. Mollo, D. Montanaro, J. Ortea and G. Cimino, J. Chem. Ecol., 2000, 26, 1563 Search PubMed.
- A. Fontana, M. L. Ciavatta, E. Mollo, C. G. Naik, S. Wahidulla, L. D'Souza and G. Cimino, J. Nat. Prod., 1999, 62, 931 CrossRef CAS.
- G. Cimino, A. Crispino, V. Di Marzo, M. Gavagin and J. D. Ros, Experientia, 1990, 46, 767 Search PubMed.
- I. Mancini, G. Guella and F. Pietra, Helv. Chim. Acta, 2000, 83, 694 CrossRef CAS.
- X. Fu, E. P. Hong and F. J. Schmitz, Tetrahedron, 2000, 56, 8989 CrossRef CAS.
- D. C. Manker, D. J. Faulkner, T. J. Stout and J. Clardy, J. Org. Chem., 1989, 54, 5371 CrossRef CAS.
- D. J. Brecknell, L. A. Collett, M. T. Davies-Coleman, M. J. Garson and D. D. Jones, Tetrahedron, 2000, 56, 2497 CrossRef CAS.
- I. Paterson, D. Y.-K. Chen, J. L. Aceña and A. S. Franklin, Org. Lett., 2000, 2, 1513 CrossRef CAS.
- G. Cimino, A. Spinella and G. Sodano, Tetrahedron Lett., 1989, 30, 3589 CrossRef CAS.
- A. Fürstner and K. Grela, Angew. Chem., Int. Ed., 2000, 39, 1234 CrossRef CAS.
- A. Fontana, P. Cavaliere, S. Wahidulla, C. G. Naik and G. Cimino, Tetrahedron, 2000, 56, 7305 CrossRef CAS.
- G. Sodano and A. Spinella, Tetrahedron Lett., 1986, 27, 2505 CrossRef CAS.
- A. Giordano, C. Della Monica, F. Landi, A. Spinella and G. Sodano, Tetrahedron Lett., 2000, 41, 3979 CrossRef CAS.
- J. A. Roesener and P. J. Scheuer, J. Am. Chem. Soc., 1986, 108, 846 CrossRef CAS.
- S. K. Chattopadhyay and G. Pattenden, J. Chem. Soc., Perkin Trans. 1, 2000, 2429 RSC.
- A. Spinella, L. A. Alvarez and G. Cimino, Tetrahedron Lett., 1998, 39, 2005 CrossRef CAS.
- I. Izzo, S. De Caro, F. De Riccardis and A. Spinella, Tetrahedron Lett., 2000, 41, 3975 CrossRef CAS.
- A. Fontana, E. Mollo, J. Ortea, M. Gavagnin and G. Cimino, J. Nat. Prod., 2000, 63, 527 CrossRef CAS.
- J. Hellou, J. E. Thompson and R. J. Andersen, Tetrahedron, 1982, 38, 1875 CrossRef CAS.
- A. T. Anilkumar, U. Sudhir, S. Joly and M. S. Nair, Tetrahedron, 2000, 56, 1899 CrossRef.
- H. Akita, M. Nozawa, A. Mitsuda and H. Ohsawa, Tetrahedron: Asymmetry, 2000, 11, 1375 CrossRef CAS.
- N. Fusetani, H. J. Wolstenholme, S. Matsunaga and H. Hirota, Tetrahedron Lett., 1991, 32, 7291 CrossRef CAS.
- T.-L. Ho, L.-R. Kung and R.-J. Chein, J. Org. Chem., 2000, 65, 5774 CrossRef CAS.
- K. Gustafson and R. J. Andersen, Tetrahedron, 1985, 41, 1101 CrossRef CAS.
- N. Ungur, M. Gavagnin, A. Fontana and G. Cimino, Tetrahedron, 2000, 56, 2503 CrossRef CAS.
- N. Takada, M. Iwatsuki, K. Suenaga and D. Uemura, Tetrahedron Lett., 2000, 41, 6425 CrossRef CAS.
- H. Kigoshi, K. Kanematsu, K. Yokota and D. Uemura, Tetrahedron, 2000, 56, 9063 CrossRef CAS.
- P. Ciminiello, E. Fattorusso, M. Forino, R. Poletti and R. Viviani, Eur. J. Org. Chem., 2000, 291 CrossRef CAS.
- A. Morohashi, M. Satake, Y. Oshima and T. Yasumoto, Biosci., Biotechnol. Biochem., 2000, 64, 1761 Search PubMed.
- T. A. Dang, R. J. Day and D. M. Hercules, Anal. Chem., 1984, 56, 866 CrossRef CAS.
- C. Vagias, C. Tsitsimpikou, T. Rapti and V. Roussis, Nat. Prod. Lett., 2000, 14, 425 Search PubMed.
- A. Loukaci, V. Bultel-Poncé, A. Longeon and M. Guyot, J. Nat. Prod., 2000, 63, 799 CrossRef CAS.
- A. Fontana, M. C. González, M. Gavagnin, J. Templado and G. Cimino, Tetrahedron Lett., 2000, 41, 429 CrossRef CAS.
- R. Durán, E. Zubía, M. J. Ortega, S. Naranjo and J. Salvá, Tetrahedron, 2000, 56, 6031 CrossRef CAS.
- M. T. Davies-Coleman, C. L. Cantrell, K. R. Gustafson, J. A. Beutler, L. K. Pannell and M. R. Boyd, J. Nat. Prod., 2000, 63, 1411 CrossRef CAS.
- A. Aiello, S. Carbonelli, G. Esposito, E. Fattorusso, T. Iuvone and M. Menna, J. Nat. Prod., 2000, 63, 1590 CrossRef CAS.
- S. S. Mitchell, D. Rhodes, F. D. Bushman and D. J. Faulkner, Org. Lett., 2000, 2, 1605 CrossRef CAS.
- N. U. Sata and N. Fusetani, Tetrahedron Lett., 2000, 41, 489 CrossRef CAS.
- M. Ishibashi, Y. Ohizumi, T. Sasaki, H. Nakamura, Y. Hirata and J. Kobayashi, J. Org. Chem., 1987, 52, 450 CrossRef CAS.
- A. J. Freyer, A. D. Patil, L. Killmer, N. Troupe, M. Mentzer, B. Carte, L. Faucette and R. K. Johnson, J. Nat. Prod., 1997, 60, 986 CrossRef CAS.
- D. Ma and H. Sun, J. Org. Chem., 2000, 65, 6009 CrossRef CAS.
- J. Kubanek, D. E. Williams, E. D. de Silva, T. Allen and R. J. Andersen, Tetrahedron Lett., 1995, 36, 6189 CrossRef CAS.
- T. Ozawa, S. Aoyagi and C. Kibayashi, Org. Lett., 2000, 2, 2955 CrossRef CAS.
- H. McAlonan, D. Potts, P. J. Stevenson and N. Thompson, Tetrahedron Lett., 2000, 41, 5411 CrossRef CAS.
- H. Takahata and N. Okamoto, Bioorg. Med. Chem. Lett., 2000, 10, 1799 CrossRef CAS.
- M. F. Raub, J. H. Cardellina II and T. F. Spande, Tetrahedron Lett., 1992, 33, 2257 CrossRef CAS.
- A. D. Patil, A. J. Freyer, R. Reichwein, B. Carte, L. B. Killmer, L. Faucette, R. K. Johnson and D. J. Faulkner, Tetrahedron Lett., 1997, 38, 363 CrossRef CAS.
- J. F. Biard, S. Guyot, C. Roussakis, J. F. Verbist, J. Vercauteren, J. F. Weber and K. Boukef, Tetrahedron Lett., 1994, 35, 2691 CrossRef CAS.
- H. Abe, S. Aoyagi and C. Kibayashi, Tetrahedron Lett., 2000, 41, 1205 CrossRef CAS.
- H. Abe, S. Aoyagi and C. Kibayashi, J. Am. Chem. Soc., 2000, 122, 4583 CrossRef CAS.
- K. Shimbo, M. Tsuda, E. Fukushi, J. Kawabata and J. Kobayashi, Tetrahedron, 2000, 56, 7923 CrossRef CAS.
- M. J. Ortega, E. Zubía, J. M. Ocaña, S. Naranjo and J. Salvá, Tetrahedron, 2000, 56, 3963 CrossRef CAS.
- A. Rudi, T. Evan, M. Aknin and Y. Kashman, J. Nat. Prod., 2000, 63, 832 CrossRef CAS.
- A. Rudi, I. Goldberg, Z. Stein, F. Frolow, Y. Benayahu, M. Schleyer and Y. Kashman, J. Org. Chem., 1994, 59, 999 CrossRef CAS.
- E. M. Beccalli, F. Clerici and A. Marchesini, Tetrahedron, 2000, 56, 2699 CrossRef CAS.
- H. Kang and W. Fenical, J. Org. Chem., 1997, 62, 3254 CrossRef CAS.
- D. L. Boger, D. R. Soenen, C. W. Boyce, M. P. Hedrick and Q. Jin, J. Org. Chem., 2000, 65, 2479 CrossRef CAS.
- A. R. Carroll, B. F. Bowden and J. C. Coll, Aust. J. Chem., 1993, 46, 489 CAS.
- C. Peschko, C. Winklhofer and W. Steglich, Chem. Eur. J., 2000, 6, 1147–1152 CrossRef CAS.
- L. H. Franco, E. B. K. Joffé, L. Puricelli, M. Tatian, A. M. Seldes and J. A. Palermo, J. Nat. Prod., 1998, 61, 1130 CrossRef CAS.
- P. M. Fresneda, P. Molina, S. Delgado and J. A. Bleda, Tetrahedron Lett., 2000, 41, 4777 CrossRef CAS.
- B. Jiang and C. Yang, Heterocycles, 2000, 53, 1489 Search PubMed.
- R. G. S. Berlinck, R. Britton, E. Piers, L. Lim, M. Roberge, R. M. da Rocha and R. J. Andersen, J. Org. Chem., 1998, 63, 9850 CrossRef CAS.
- E. Piers, R. Britton and R. J. Andersen, J. Org. Chem., 2000, 65, 530 CrossRef CAS.
- H. Sato, M. Tsuda, K. Watanabe and J. Kobayashi, Tetrahedron, 1998, 54, 8687 CrossRef CAS.
- P. M. Fresneda, P. Molina and M. A. Sanz, Synlett, 2000, 1190 CAS.
- A. Garcia, L. A. Lenis, C. Jiménez, C. Debitus, E. Quiñoá and R. Riguera, Org. Lett., 2000, 2, 2765 CrossRef CAS.
- N. Bontemps, I. Bonnard, B. Banaigs, G. Combaut and C. Francisco, Tetrahedron Lett., 1994, 35, 7023 CrossRef CAS.
- E. Delfourne, N. Bontemps-Subielos and J. Bastide, Tetrahedron Lett., 2000, 41, 3863 CrossRef CAS.
- G. Koren-Goldschlanger, M. Aknin and Y. Kashman, J. Nat. Prod., 2000, 63, 830 CrossRef.
- A. Plubrukarn and B. S. Davidson, J. Org. Chem., 1998, 63, 1657–1659 CrossRef CAS.
- E. Delfourne, C. Roubin and J. Bastide, J. Org. Chem., 2000, 65, 5476 CrossRef CAS.
- J. Kobayashi, J. Cheng, H. Nakamura, Y. Ohizumi, Y. Hirata, T. Sasaki, T. Ohta and S. Nozoe, Tetrahedron Lett., 1988, 29, 1177 CrossRef CAS.
- F. J. Schmitz, F. S. De Guzman, M. B. Hossain and D. van der Helm, J. Org. Chem., 1991, 56, 804 CrossRef CAS.
- M. Álvarez, L. Feliu, W. Ajana, J. A. Joule and J. L. Fernández-Puentes, Eur. J. Org. Chem., 2000, 849 CrossRef CAS.
- M. Álvarez, L. Feliu, W. Ajana and J. A. Joule, Tetrahedron, 2000, 56, 3703 CrossRef CAS.
- B. S. Lindsay, H. C. Christiansen and B. R. Copp, Tetrahedron, 2000, 56, 497 CrossRef CAS.
- A. Aiello, E. Fattorusso, M. Menna and T. Iuvone, J. Nat. Prod., 2000, 63, 517 CrossRef CAS.
- L. A. Morris, J. J. Kettenes van den Bosch, K. Versluis, G. S. Thompson and M. Jaspars, Tetrahedron, 2000, 56, 8345 CrossRef CAS.
- X. Fu, J. Su and L. Xeng, Sci. China, Ser. B: Chem., 2000, 43, 643 Search PubMed.
- S. G. Toske and W. Fenical, Tetrahedron Lett., 1995, 36, 8355 CrossRef CAS.
- C. D. J. Boden, M. Norley and G. Pattenden, J. Chem. Soc., Perkin Trans. 1, 2000, 883 RSC.
- F. J. Schmitz, M. B. Ksebati, J. S. Chang, J. L. Wang, M. B. Hossain, D. van der Helm, M. H. Engel and A. Serban, J. Org. Chem., 1989, 54, 3463 CrossRef CAS.
- C. D. J. Boden and G. Pattenden, J. Chem. Soc., Perkin Trans. 1, 2000, 875 RSC.
- A. R. Carroll, J. C. Coll, D. J. Bourne, J. K. MacLeod, T. M. Zabriskie, C. M. Ireland and B. F. Bowden, Aust. J. Chem., 1996, 49, 659.
- P. Wipf and Y. Uto, J. Org. Chem., 2000, 65, 1037 CrossRef CAS.
- H. Vervoort, W. Fenical and R. de A. Epifanio, J. Org. Chem., 2000, 65, 782 CrossRef CAS.
- M. M. Joullié, P. Portonovo, B. Liang and D. J. Richard, Tetrahedron Lett., 2000, 41, 9373 CrossRef CAS.
- B. Liang, P. Portonovo, M. D. Vera, D. Xiao and M. M. Joullié, Org. Lett., 1999, 1, 1319 CrossRef CAS.
- J. A. Tincu, A. G. Craig and S. W. Taylor, Biochem. Biophys. Res. Commun., 2000, 270, 421 CrossRef CAS.
- E. Fahy, B. C. M. Potts, D. J. Faulkner and K. Smith, J. Nat. Prod., 1991, 54, 564 CrossRef CAS.
- A. E. Wright, D. A. Forleo, G. P. Gunawardana, S. P. Gunasekera, F. E. Koehn and O. J. McConnell, J. Org. Chem., 1990, 55, 4508 CrossRef CAS.
- K. L. Rinehart, T. G. Holt, N. L. Fregeau, J. G. Stroh, P. A. Keifer, F. Sun, L. H. Li and D. G. Martin, J. Org. Chem., 1990, 55, 4512 CrossRef CAS.
- C. Cuevas, M. Pérez, M. J. Martín, J. L. Chicharro, C. Fernández-Rivas, M. Flores, A. Francesch, P. Gallego, M. Zarzuelo, F. de la Calle, J. García, C. Polanco, I Rodríguez and I. Manzanares, Org. Lett., 2000, 2, 2545 CrossRef CAS.
- K. L. Rinehart, Jr., J. Kobayashi, G. C. Harbour, R. G. Hughes, Jr., S. A. Mizsak and T. A. Scahill, J. Am. Chem. Soc., 1984, 106, 1524 CrossRef.
- J.-J. Liu, T. Hino, A. Tsuruoka, N. Harada and M. Nakagawa, J. Chem. Soc., Perkin Trans. 1, 2000, 3487 RSC.
- B. R. Copp, C. A. Wassvik, G. Lambert and M. J. Page, J. Nat. Prod., 2000, 63, 1168 CrossRef CAS.
- T. Miyamoto, A. Yamamoto, M. Wakayabashi, Y. Nagaregawa, M. Inagaki, R. Higuchi, M. Iha and K. Teruya, Eur. J. Org. Chem., 2000, 2295 CrossRef CAS.
- G. P. Smirnova, Russ. Chem. Bull., 2000, 49, 165 Search PubMed.
- G. P. Smirnova, Russ. Chem. Bull., 2000, 49, 159 Search PubMed.
- K. Yamada, Y. Harada, T. Miyamoto, R. Isobe and R. Higuchi, Chem. Pharm. Bull., 2000, 48, 157 Search PubMed.
- Y. Murata and N. U. Sata, J. Agric. Food Chem., 2000, 48, 5557 CrossRef CAS.
- N. Takahashi, M. Ojika and D. Ejima, Fish. Sci., 2000, 66, 412 Search PubMed.
- N. V. Ivanchina, A. A. Kicha, A. I. Kalinovsky, P. S. Dmitrenok, V. A. Stonik, R. Riguera and C. Jiménez, J. Nat. Prod., 2000, 63, 1178 CrossRef CAS.
- A. A. Kicha, N. V. Ivanchina, A. I. Kalinovsky, P. S. Dmitenok and V. A. Stonik, Russ. Chem. Bull., 2000, 49, 1794 Search PubMed.
- M. E. Diaz de Vivar, M. S. Maier and A. M. Seldes, Anal. Asoc. Quim. Arg., 1999, 87, 247 Search PubMed.
- S. De Marino, M. Iorizzi, F. Zollo, C. D. Amsler, S. P. Greer and J. B. McClintock, Eur. J. Org. Chem., 2000, 4093 CrossRef CAS.
- M. Sandvoss, L. H. Pham, K. Levsen, A. Preiss, C. Mügge and G. Wünsch, Eur. J. Org. Chem., 2000, 1253 CrossRef CAS.
- S. A. Avilov, A. S. Antonov, O. A. Drozdova, V. I. Kalinin, A. I. Kalinovsky, V. A. Stonik, R. Riguera, L. A. Lenis and C. Jiménez, J. Nat. Prod., 2000, 63, 65 CrossRef CAS.
- S. A. Avilov, A. S. Antanov, O. A. Drozdova, V. I. Kalinin, A. I. Kalinovsky, R. Riguera, L. A. Lenis and C. Jiménez, J. Nat. Prod., 2000, 63, 1349 CrossRef CAS.
- S. H. Kim, S.-M. Yoo, I. S. Park and Y. H. Kim, J. Nat. Prod., 2000, 63, 1188 CrossRef CAS.
- N. Asai, N. Fusetani, S. Matsunaga and J. Sasaki, Tetrahedron, 2000, 56, 9895 CrossRef CAS.
- Y. Kishi, T. Goto, S. Eguchi, Y. Hirata, E. Watanabe and T. Aoyama, Tetrahedron Lett., 1966, 3437 CrossRef CAS.
- H. Nakamura, M. Aizawa, D. Takeuchi, A. Murai and O. Shimoura, Tetrahedron Lett., 2000, 41, 2185 CrossRef CAS.
- T. Higa, R. K. Okuda, R. M. Severns, P. J. Scheuer, C.-H. He, C. Xu and J. Clardy, Tetrahedron, 1987, 43, 1063 CrossRef CAS.
- O. Block, G. Klein, H.-J. Altenbach and D. J. Brauer, J. Org. Chem., 2000, 65, 716 CrossRef CAS.
- H. Onuki, K. Tachibana and N. Fusetani, Tetrahedron Lett., 1993, 34, 5609 CrossRef CAS.
- A. Fujiwara, T. Kan and T. Fukuyama, Synlett, 2000, 11, 1667–1669.
- M. N. Rao, A. E. Shinnar, L. A. Noecker, T. L. Chao, B. Feibush, B. Snyder, I. Sharkansky, A. Sarkahian, X. Zhang, S. R. Jones, W. A. Kinney and M. Zasloff, J. Nat. Prod., 2000, 63, 631 CrossRef CAS.
- K. A. Francesconi, S. Khokiattiwong, W. Goessler, S. N. Pedersen and M. Pavkov, Chem. Commun., 2000, 1083 RSC.
- N. S. Reddy and Y. Venkateswarlu, Indian J. Chem., Sect B: Org. Chem. Incl. Med. Chem., 2000, 39, 971 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |