Hetero-binuclear complexes containing a Ru0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) Mn+ bond bridged by P,N-phosphine ligands: convenient synthesis of tridentate organometallic trans-Ru(CO)3(L)2
Mn+ bond bridged by P,N-phosphine ligands: convenient synthesis of tridentate organometallic trans-Ru(CO)3(L)2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) (L
(L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) phosphine bearing an N-donor substituent) ligands
phosphine bearing an N-donor substituent) ligands
Hai-Bin
Song
a,
Zheng-Zhi
Zhang
b and
Thomas
C. W. Mak
*a
aDepartment of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR, P. R. China. E-mail: tcwmak@cuhk.edu.hk; Fax: +852 2603-5057
bState Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, P. R. China
First published on 9th January 2002
Abstract
A convenient new synthesis of trans-Ru(CO)3(μ-L)2
[L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-(diphenylphosphino)pyridine, N-(diphenylphosphinomethyl)morpholine)] was developed. The reactions of these two tridentate organometallic ligands with selected group 11 and 12 metal salts resulted in the formation of hetero-binuclear Ru0
2-(diphenylphosphino)pyridine, N-(diphenylphosphinomethyl)morpholine)] was developed. The reactions of these two tridentate organometallic ligands with selected group 11 and 12 metal salts resulted in the formation of hetero-binuclear Ru0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn+
[M
Mn+
[M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag(I), n
Ag(I), n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; Hg(II), Cd(II), n
1; Hg(II), Cd(II), n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2] complexes with Ru–Ag
2] complexes with Ru–Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7132(7)
Å, Ru–Hg
2.7132(7)
Å, Ru–Hg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7075(4)
Å and Ru–Cd
2.7075(4)
Å and Ru–Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7750(9)
Å, as determined by X-ray crystallography.
2.7750(9)
Å, as determined by X-ray crystallography.
Binuclear complexes containing a metal–metal bond have been studied intensively due to their interesting structural features, spectral properties and wide applications.1 The formation of a metal–metal bond depends on two factors: (i) a favorable Lewis acid-base relationship between the two metals and (ii) suitability of the bridging ligands. Most binuclear complexes are stabilized through a bridging ligand with short dentate separation, but unsupported metal–metal dative bonds can also be formed.2 The hemilabile bridging ligand 2-(diphenylphosphino)pyridine, Ph2Ppy, has been widely used to coordinate different metals.3 Due to the presence of its basic nitrogen site that acts as an effective “proton messenger”, the monodentate P-coordination mode of Ph2Ppy plays a crucial role in the rhodium-catalyzed hydroformylation of styrene and palladium-catalyzed carbonylation of alkynes.3e–g On the other hand, due to electronic differentiation between the nitrogen and phosphorus sites, this ligand readily coordinates different metals to form hetero-binuclear complexes, some of which exhibit interesting luminescent properties.1b,3 In some catalytic applications, the synergistic effect confers to the hetero-binuclear complexes higher activities and conversion rates as compared to the mononuclear complexes.3
Binuclear complexes containing a ruthenium carbonyl group and a metal–metal dative bond are scarce as there exists no efficient method of preparing mononuclear P-coordinated ruthenium(0) carbonyl compounds.4 Here we report a new procedure to synthesize trans-Ru(CO)3(Ph2Ppy)2 whose hetero-binuclear complex with silver(I) is also described.
Thus far, most P,N-phosphine ligands used in the synthesis of metal–metal bonded binuclear complexes have been rigid pyridylphosphine ligands with a short P,N-donor separation. The paucity of aminophosphine ligands functioning in the bidentate bridging mode is due to (i) the nearly planar configuration around the amino nitrogen atom in a primary amine, secondary amine or aromatic amine and (ii) the low Lewis basicity of a tertiary amine, which does not favor its coordination to a metal center. Our previous work has established that the tertiary amino nitrogen atom in a cylclohexane-like ring such as piperazine5a or morpholine5b has a pyramidal configuration that ensures its efficient coordination to a metal atom. Hence we employed the non-rigid phosphine ligand N-(diphenylphosphinomethyl)morpholine, Ph2PCH2morph, to prepare the new tridentate organometallic ligand trans-Ru(CO)3(Ph2PCH2morph)2, whose reactivity and formation of hetero-binuclear complexes with mercury(II) and cadmium(II) salts are also reported.
Experimental
Unless otherwise stated, all reactions were performed under a nitrogen atmosphere using Schlenk techniques. The solvents were purified by standard methods. Infrared spectra were recorded on a Shimadzu 435 spectrometer as KBr discs. The 31P{1H} NMR spectra were recorded on a JEOL EX270 spectrometer at 109.25 MHz using H3PO4 as the external standard and CDCl3 as solvent. Ru3(CO)12 (Strem) was used as received, as were AgOTf (Acros), HgI2 (Strem), CdI2 (Strem) and Ph2PH (Aldrich). The ligand 2-(diphenylphosphino)pyridine was prepared by the literature method.3aSynthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and stirred at this temperature for 24 h to form a clear solution. After cooling, the solution was filtered through a 2 cm celite pad, and the solvent was removed from the filtrate in vacuo. EtOH (50 ml) was added to the oily residue and the solution was cooled to −20
°C and stirred at this temperature for 24 h to form a clear solution. After cooling, the solution was filtered through a 2 cm celite pad, and the solvent was removed from the filtrate in vacuo. EtOH (50 ml) was added to the oily residue and the solution was cooled to −20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C overnight. Colorless micro-crystals of Ph2PCH2morph were collected by filtration in 65%
yield. m.p. 53–55 C; 31P{1H}NMR: δ
°C overnight. Colorless micro-crystals of Ph2PCH2morph were collected by filtration in 65%
yield. m.p. 53–55 C; 31P{1H}NMR: δ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −27.1.
−27.1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Then cut pieces of sodium metal (6 mmol) were introduced and the colorless solution immediately turned blue. The solution was stirred continuously for 2 h at −50
°C. Then cut pieces of sodium metal (6 mmol) were introduced and the colorless solution immediately turned blue. The solution was stirred continuously for 2 h at −50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C , during which time the color changed to pale yellow; liquid ammonia was then allowed to slowly evaporate. After the residue was further dried in vacuum to remove the residual ammonia, a mixture of 20 cm3 EtOH with 0.15 g (1.5 mmol) concentrated H2SO4
(98%) was added and the solution stirred for 2 min. The phosphine ligand Ph2Ppy (6 mmol) was next introduced and the solution heated to 70
°C , during which time the color changed to pale yellow; liquid ammonia was then allowed to slowly evaporate. After the residue was further dried in vacuum to remove the residual ammonia, a mixture of 20 cm3 EtOH with 0.15 g (1.5 mmol) concentrated H2SO4
(98%) was added and the solution stirred for 2 min. The phosphine ligand Ph2Ppy (6 mmol) was next introduced and the solution heated to 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 2 h, during
which time a large amount of yellow precipitate appeared. After the reaction was complete, the solution was cooled to room temperature and the precipitate was filtered. The solid was dissolved in 20 cm3 CH2Cl2, filtered, and concentrated to 5 cm3. Then 15 cm3 CH3OH was added and the solution cooled in a refrigerator overnight. Pure product 1 appeared as a pale yellow precipitate and was collected by filtration and vacuum dried. Yield: 50%. The ν(CO) IR and 31P NMR spectra were identical to those reported in the literature.4 IR (νCO): 1897s cm−1; 31P{1H} NMR: δ
°C for 2 h, during
which time a large amount of yellow precipitate appeared. After the reaction was complete, the solution was cooled to room temperature and the precipitate was filtered. The solid was dissolved in 20 cm3 CH2Cl2, filtered, and concentrated to 5 cm3. Then 15 cm3 CH3OH was added and the solution cooled in a refrigerator overnight. Pure product 1 appeared as a pale yellow precipitate and was collected by filtration and vacuum dried. Yield: 50%. The ν(CO) IR and 31P NMR spectra were identical to those reported in the literature.4 IR (νCO): 1897s cm−1; 31P{1H} NMR: δ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.1.
50.1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.8.
16.8.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59.4.
59.4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34.4, 2J(199Hg, 31P)
34.4, 2J(199Hg, 31P)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 Hz. Re-crystallization of 4 in CHCl3–Et2O
yielded yellow crystals of 4·Et2O for X-ray analysis.
123 Hz. Re-crystallization of 4 in CHCl3–Et2O
yielded yellow crystals of 4·Et2O for X-ray analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.9, 2J(111Cd, 31P)
36.9, 2J(111Cd, 31P)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 Hz. Re-crystallization of 5
in CHCl3–Et2O yielded well-formed yellow crystals of 5·Et2O for X-ray analysis.
28 Hz. Re-crystallization of 5
in CHCl3–Et2O yielded well-formed yellow crystals of 5·Et2O for X-ray analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Anal found: C, 54.05; H, 4.93; N, 3.34; calcd for C36H40N2O4Cl2P2Ru:
C, 54.14; H, 5.05; N, 3.51. IR (νCO): 2050s, 1987s cm−1; 31P{1H} NMR: δ
°C. Anal found: C, 54.05; H, 4.93; N, 3.34; calcd for C36H40N2O4Cl2P2Ru:
C, 54.14; H, 5.05; N, 3.51. IR (νCO): 2050s, 1987s cm−1; 31P{1H} NMR: δ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21.2.
21.2.
X-Ray crystallography
For complexes 3 and 4·Et2O, a selected single crystal was mounted on a Bruker SMART 1000 CCD diffractometer operating at 50 kV and 30 mA using Mo-Kα radiation (0.71073 Å). Data collection at 293 K and reduction were performed using SMART and SAINT software,6 with frames of 0.3° oscillation in the range 1.5°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) <
<![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ
θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) <
<![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28°. An empirical absorption correction was applied using the SADABS program.7
28°. An empirical absorption correction was applied using the SADABS program.7
The data for 5·Et2O was collected at 293 K on a Rigaku RAXIS IIC imaging plate diffractometer using Mo-Kα radiation from a rotating anode generator operating at 50 kV and 90 mA, 36/5° oscillation frames in the range of 0–180°, and exposure of 8 min per frame. A self-consistent semi-empirical absorption correction based on Fourier coefficient fitting of symmetry-equivalent reflections was applied using the ABSCOR program.8
The data for 6·CH2Cl2 and 6·0.5CH2Cl2 were collected at 293 K in the variable ω-scan mode on a Siemens R3m/V four-circle diffractometer using Mo-Kα radiation (50 kV, 30 mA; 2θmax![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50° for 6·CH2Cl2 and 52° for 6·0.5CH2Cl2). An empirical absorption correction was applied using ψ-scan data.
50° for 6·CH2Cl2 and 52° for 6·0.5CH2Cl2). An empirical absorption correction was applied using ψ-scan data.
All structures were solved by direct methods and all non-hydrogen atoms were subjected to anisotropic refinement by full-matrix least-squares on F2 using the SHELXTL package.9 Non-hydrogen atoms were subjected to anisotropic refinement. All hydrogen atoms were generated geometrically (C–H bond lengths fixed at 0.96 Å), assigned appropriate isotropic thermal parameters, and included in structure factor calculations in the final stage of F2 refinement. Selected X-ray data are given in Table 1.
a
R1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Σ(|Fo| Σ(|Fo|![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |Fc|)/Σ|Fo|; wR2 |Fc|)/Σ|Fo|; wR2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) {w[Σ(|Fo| {w[Σ(|Fo|![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |Fc|)2]/Σ|Fo|2}1/2. |Fc|)2]/Σ|Fo|2}1/2.
|
|||||
|---|---|---|---|---|---|
| 3 | 4·Et2O | 5·Et2O | 6·CH2Cl2 | 6·0.5CH2Cl2 | |
| Formula | C38H28AgF3N2O6P2RuS | C37H40HgI2N2O5P2Ru·Et2O | C37H40CdI2N2O5P2Ru·Et2O | C36H40Cl2N2O4P2Ru·CH2Cl2 | C36H40Cl2N2O4P2Ru·0.5CH2Cl2 |
| M | 968.56 | 1284.23 | 1196.04 | 883.54 | 841.07 |
| Crystal system | Triclinic | Monoclinic | Monoclinic | Triclinic | Tetragonal |
| Space group | P 1 (no. 2) | P21/c (no. 14) | P21/c (no. 14) | P 1 (no. 2) | I41/a (no. 88) |
| a/Å | 11.170(2) | 14.6934(8) | 14.682(3) | 11.062(2) | 20.928(2) |
| b/Å | 11.430(2) | 12.7794(7) | 12.739(3) | 11.362(2) | 20.928(2) |
| c/Å | 18.240(3) | 24.918(1) | 24.921(5) | 18.146(4) | 17.798(4) |
| α/° | 75.620(3) | 90 | 90 | 95.48(2) | 90 |
| β/° | 74.322(3) | 91.410(1) | 91.54(3) | 94.42(2) | 90 |
| γ/° | 64.449(3) | 90 | 90 | 113.05(1) | 90 |
| U/Å3 | 1999.5(5) | 4677.4(4) | 4659.4(16) | 2072.8(7) | 7795(2) |
| Z | 2 | 4 | 4 | 2 | 8 |
| μ(Mo-Kα)/mm−1 | 1.060 | 5.033 | 2.218 | 0.752 | 0.730 |
| Total reflect. | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093 093 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 692 692 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 197 197 |
8671 | 6595 |
| Unique reflect. (Rint) | 9437 (0.0379) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 (0.0403 ) 260 (0.0403 ) |
7730 (0.0704) | 7300 (0.0538) | 2914 (0.0490) |
| Obsd reflect. | 4764 | 7303 | 6754 | 4132 | 1910 |
R1, wR2 [I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) > >![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2σ(I)]a 2σ(I)]a |
0.0553, 0.1187 | 0.0311, 0.0602 | 0.0601, 0.1605 | 0.0670, 0.1238 | 0.0487, 0.1274 |
| R1, wR2 (all data) | 0.1287, 0.1501 | 0.0628, 0.0670 | 0.0702, 0.1688 | 0.1363, 0.1531 | 0.0893, 0.1464 |
CCDC reference numbers 169883–169887. See http://www.rsc.org/suppdata/nj/b1/b108287f/ for crystallographic data in CIF or other electronic format.
Results and discussion
Over the years, the synthesis of trans-Ru(CO)3(L)2 (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphine ligand) has been developed in several ways. For instance:
phosphine ligand) has been developed in several ways. For instance: - (ii) Redox reaction:10a

(3)
- (iii) Photochemical reaction:4a

(4)
It is not convenient to use Ru(CO)3(cod) in the synthesis because it slowly decomposes, even when stored at −20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.10b,c The second method is very useful for phosphine ligands that contain no basic and heat-sensitive functional groups, but it is not applicable if the ligand contains a N-donor atom, which would preferentially coordinate to the Ru atom at the expense of the desired P-coordination. The third method is a photochemical process using ultra-violet radiation, and the optimum reaction conditions change with different ligands. In cases where the phosphine ligand bears a photo-sensitive group such as anthracene, it is not workable. Clearly, there is a need for a new convenient procedure that makes use of commercially available chemicals as starting materials.
°C.10b,c The second method is very useful for phosphine ligands that contain no basic and heat-sensitive functional groups, but it is not applicable if the ligand contains a N-donor atom, which would preferentially coordinate to the Ru atom at the expense of the desired P-coordination. The third method is a photochemical process using ultra-violet radiation, and the optimum reaction conditions change with different ligands. In cases where the phosphine ligand bears a photo-sensitive group such as anthracene, it is not workable. Clearly, there is a need for a new convenient procedure that makes use of commercially available chemicals as starting materials.
It is noted that a selective synthesis of the iron analog trans-Fe(CO)3(PR3)2 was reported in the late 1980's by Keiter and Brunet and their coworkers,11 who used Fe(CO)5 to react with KOH (or NaOH, or NaBH4) and different types of phosphine ligands PR3 in refluxing n-butanol or ethanol to obtain the product in high yield. The key intermediate of this reaction is [HFe(CO)4]−, which suggests to us that an analogous reaction with [HRu(CO)4]− as an intermediate might be feasible. However, unlike its iron analog Fe(CO)5, Ru(CO)5 is rather unstable and cannot be used as a starting material. On the other hand, it is known that Ru3(CO)12 reacts with a solution of sodium metal
in liquid ammonia to form Na2[Ru(CO)4].12 Accordingly, we proceeded to carry out the reaction as represented by the following equation and obtained trans-Ru(CO)3(L)2
(L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P,N-phosphine ligand) in good yield:
P,N-phosphine ligand) in good yield:

| (5) |
The key intermediate [HRu(CO)4]− is more reactive than [HFe(CO)4]−, as the reaction time of 2 h for the ruthenium complex is 12 times less than that required for the iron complex. It is worthy of note that an attempt to prepare the osmium analog using Os3(CO)12 by the same procedure was unsuccessful, though the reason is unclear.
The reaction of ligands 1 and 2 with different metal salts is shown in Scheme 1. Ligand 1 has been employed to react with group 12 metal salts in CH2Cl2 to form binuclear compounds that feature a weak Ru–Zn bond, a strong Ru–Hg bond, and strong Ru–Cd bonds.4b However, using copper(I) and silver(I) salts under the same conditions resulted in a redox reaction in which 1 suffered decomposition and the metal(I) ions were reduced to metallic powder. This phenomenon is similar to that observed in the reaction of Fe(CO)3(PR3)2 with silver salts {which yield metallic silver and the unstable cationic radical [Fe(CO)3(PR3)2]+}13 and indicates that the [Ru(CO)3(Ph2Ppy)2]+ radical is also formed. On the other hand, it has been reported that silver salts are less exoergic in THF than in CH2Cl2.14 So we carried out the reaction in THF instead of CH2Cl2 and obtained the Ru–Ag binuclear complex, although the Ru–Cu complex proved to be unstable and was not isolated.
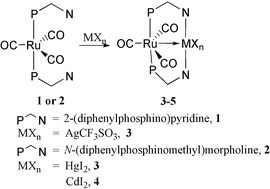 | ||
| Scheme 1 | ||
According to our previous findings,5b,15 when the organometallic ligand trans-M(CO)3(PR3)2 coordinates with another metal to form a binuclear complex, (i) the local symmetry of M changes from D3h to C2v, which causes ν(CO) splitting, and (ii) the formation of a metal–metal dative bond decreases the electron density at M and consequently the dπ(M)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →
→![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) π*(CO)
π-back bonding, which results in an increase of ν(CO) with respect to the free ligand. As expected, the ν(CO) IR spectrum of binuclear complex 3 shows both characteristics. The 31P NMR spectrum of complex 3 exhibits a singlet at 59.4 ppm, indicating that the two phosphine moieties are chemically equivalent.
π*(CO)
π-back bonding, which results in an increase of ν(CO) with respect to the free ligand. As expected, the ν(CO) IR spectrum of binuclear complex 3 shows both characteristics. The 31P NMR spectrum of complex 3 exhibits a singlet at 59.4 ppm, indicating that the two phosphine moieties are chemically equivalent.
The molecular structure of compound 3 is depicted in Fig. 1, and selected bond lengths and angles are listed in Table 2. Complex 3 displays a distorted octahedral geometry about the Ru atom, in which both P–Ru bonds are tilted toward the silver atom with an unusually small P(1)–Ru(1)–P(2) angle of 155.53(6)°. This is rather different from what is seen in the related binuclear complexes Ru(CO)3(μ-Ph2Ppy)2MCl2
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn, Cd, Hg),4b in which the RuP2 unit is much closer to being linear [P–Ru–P angle: 169.6(1)–173.9(1)°], and in the iron analogs Fe(CO)3(μ-Ph2Ppy)2MX2
(M
Zn, Cd, Hg),4b in which the RuP2 unit is much closer to being linear [P–Ru–P angle: 169.6(1)–173.9(1)°], and in the iron analogs Fe(CO)3(μ-Ph2Ppy)2MX2
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu,
Ag, Cd, Hg; X
Cu,
Ag, Cd, Hg; X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl, Br, I, SCN, ClO4), in which the FeP2 unit is also close to linear (P–Fe–P angle: 168–174°). On the other hand, the three CO ligands and silver atom lie approximately in a plane. The silver atom is four-coordinated and adopts a distorted tetrahedral geometry with Ag–N
Cl, Br, I, SCN, ClO4), in which the FeP2 unit is also close to linear (P–Fe–P angle: 168–174°). On the other hand, the three CO ligands and silver atom lie approximately in a plane. The silver atom is four-coordinated and adopts a distorted tetrahedral geometry with Ag–N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.373(5) and 2.404(6)
Å, Ag–O
2.373(5) and 2.404(6)
Å, Ag–O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.295(6)
Å and Ru–Ag
2.295(6)
Å and Ru–Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7132(7)
Å, while the bond angles vary from 93.2(1) to 144.0(2)°. The Ru–Ag distance is smaller than the sum of the metallic radii of Ru and Ag (2.78 Å) and those in ruthenium clusters containing a Ru–Ag bond: 2.812 and 2.825 Å16a in Ru4H2(CO)12Ag(Ph3P)2·CH2Cl2,
2.730 and 2.802 Å16b in (dppm)2Ru3(CO)8Ag(CF3CO2)·0.5CH2Cl2, and 2.767 and 2.806 Å16c in (dppm)Ru3(CO)12AgPPh3. This is suggestive of the presence of a fairly strong Ru→Ag donor–acceptor bond in 3.
2.7132(7)
Å, while the bond angles vary from 93.2(1) to 144.0(2)°. The Ru–Ag distance is smaller than the sum of the metallic radii of Ru and Ag (2.78 Å) and those in ruthenium clusters containing a Ru–Ag bond: 2.812 and 2.825 Å16a in Ru4H2(CO)12Ag(Ph3P)2·CH2Cl2,
2.730 and 2.802 Å16b in (dppm)2Ru3(CO)8Ag(CF3CO2)·0.5CH2Cl2, and 2.767 and 2.806 Å16c in (dppm)Ru3(CO)12AgPPh3. This is suggestive of the presence of a fairly strong Ru→Ag donor–acceptor bond in 3.
![Perspective view (35% thermal ellipsoids) of the molecular structure of [Ru(CO)3(μ-Ph2Ppy)2AgCF3SO3], 3. In this and all other figures, the hydrogen atoms have been omitted for clarity.](/image/article/2002/NJ/b108287f/b108287f-f1.gif) | ||
| Fig. 1 Perspective view (35% thermal ellipsoids) of the molecular structure of [Ru(CO)3(μ-Ph2Ppy)2AgCF3SO3], 3. In this and all other figures, the hydrogen atoms have been omitted for clarity. | ||
a Symmetry code #1: −x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, −y 1, −y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3/2, z. 3/2, z.
|
|||
|---|---|---|---|
| 3 | |||
| Ru(1)–C(1) | 1.944(8) | Ag(1)–Ru(1) | 2.7132(7) |
| Ru(1)–C(2) | 1.926(7) | Ag(1)–N(1) | 2.373(5) |
| Ru(1)–C(3) | 1.918(7) | Ag(1)–N(2) | 2.404(6) |
| Ru(1)–P(1) | 2.367(2) | Ag(1)–O(4) | 2.295(6) |
| Ru(1)–P(2) | 2.357(2) | ||
| P(1)–Ru(1)–P(2) | 155.53(6) | P(2)–Ru(1)–Ag(1) | 78.10(4) |
| C(1)–Ru(1)–C(2) | 100.1(3) | C(1)–Ru(1)–Ag(1) | 72.3(2) |
| C(1)–Ru(1)–C(3) | 156.5(3) | C(2)–Ru(1)–Ag(1) | 172.4(2) |
| C(2)–Ru(1)–C(3) | 103.4(3) | C(3)–Ru(1)–Ag(1) | 84.2(2) |
| C(1)–Ru(1)–P(1) | 89.0(2) | O(4)–Ag(1)–Ru(1) | 144.0(2) |
| C(2)–Ru(1)–P(1) | 102.0(2) | N(1)–Ag(1)–Ru(1) | 93.9(1) |
| C(3)–Ru(1)–P(1) | 86.5(2) | N(2)–Ag(1)–Ru(1) | 93.2(1) |
| C(1)–Ru(1)–P(2) | 88.0(2) | N(1)–Ag(1)–N(2) | 110.3(2) |
| C(2)–Ru(1)–P(2) | 102.4(2) | O(4)–Ag(1)–N(1) | 105.4(2) |
| C(3)–Ru(1)–P(2) | 86.6(2) | O(4)–Ag(1)–N(2) | 107.6(2) |
| P(1)–Ru(1)–Ag(1) | 77.86(4) | ||
| 4·Et2O | |||
| Ru(1)–C(1) | 1.950(4) | Ru(1)–P(2) | 2.389(1) |
| Ru(1)–C(2) | 1.938(5) | Hg(1)–Ru(1) | 2.7075(4) |
| Ru(1)–C(3) | 1.947(5) | Hg(1)–I(1) | 2.8128(4) |
| Ru(1)–P(1) | 2.387(1) | Hg(1)–I(2) | 2.7948(4) |
| P(1)–Ru(1)–P(2) | 178.83(4) | C(2)–Ru(1)–C(3) | 101.3(2) |
| C(1)–Ru(1)–P(1) | 90.2(1) | P(1)–Ru(1)–Hg(1) | 86.55(3) |
| C(2)–Ru(1)–P(1) | 89.0(1) | P(2)–Ru(1)–Hg(1) | 93.37(3) |
| C(3)–Ru(1)–P(1) | 92.0(1) | C(1)–Ru(1)–Hg(1) | 81.3(1) |
| C(1)–Ru(1)–P(2) | 88.6(1) | C(2)–Ru(1)–Hg(1) | 175.1(1) |
| C(2)–Ru(1)–P(2) | 91.1(1) | C(3)–Ru(1)–Hg(1) | 76.9(1) |
| C(3)–Ru(1)–P(2) | 89.1(1) | Ru(1)–Hg(1)–I(2) | 127.74(1) |
| C(2)–Ru(1)–C(1) | 100.6(2) | Ru(1)–Hg(1)–I(1) | 122.57(1) |
| C(3)–Ru(1)–C(1) | 158.0(2) | I(2)–Hg(1)–I(1) | 106.62(1) |
| 5·Et2O | |||
| Ru(1)–C(1) | 1.930(8) | Ru(1)–Cd(1) | 2.7750(9) |
| Ru(1)–C(2) | 1.937(7) | Cd(1)–N(1) | 2.519(5) |
| Ru(1)–C(3) | 1.941(7) | Cd(1)–I(1) | 2.7728(8) |
| Ru(1)–P(1) | 2.372(2) | Cd(1)–I(2) | 2.7857(9) |
| Ru(1)–P(2) | 2.379(2) | ||
| P(1)–Ru(1)–P(2) | 178.64(6) | C(1)–Ru(1)–Cd(1) | 74.7(2) |
| C(1)–Ru(1)–C(2) | 103.9(3) | C(2)–Ru(1)–Cd(1) | 171.9(2) |
| C(1)–Ru(1)–C(3) | 154.1(3) | C(3)–Ru(1)–Cd(1) | 80.2(2) |
| C(2)–Ru(1)–C(3) | 101.9(3) | P(1)–Ru(1)–Cd(1) | 83.05(5) |
| C(1)–Ru(1)–P(1) | 93.0(2) | P(2)–Ru(1)–Cd(1) | 97.28(5) |
| C(2)–Ru(1)–P(1) | 89.1(2) | N(1)–Cd(1)–I(1) | 112.8(1) |
| C(3)–Ru(1)–P(1) | 90.1(2) | N(1)–Cd(1)–Ru(1) | 93.8(1) |
| C(1)–Ru(1)–P(2) | 88.4(2) | I(1)–Cd(1)–Ru(1) | 123.18(2) |
| C(2)–Ru(1)–P(2) | 90.6(2) | N(1)–Cd(1)–I(2) | 93.3(1) |
| C(3)–Ru(1)–P(2) | 88.7(2) | I(1)–Cd(1)–I(2) | 107.74(3) |
| 6·CH2Cl2 | |||
| Ru(1)–C(1) | 1.854(9) | Ru(1)–P(2) | 2.407(2) |
| Ru(1)–C(2) | 1.894(8) | Ru(1)–Cl(1) | 2.441(2) |
| Ru(1)–P(1) | 2.411(2) | Ru(1)–Cl(2) | 2.447(2) |
| C(1)–Ru(1)–C(2) | 90.6(3) | P(1)–Ru(1)–Cl(1) | 87.70(7) |
| C(1)–Ru(1)–P(1) | 96.0(2) | P(2)–Ru(1)–Cl(1) | 88.59(7) |
| C(2)–Ru(1)–P(1) | 92.4(2) | C(1)–Ru(1)–Cl(2) | 177.2(3) |
| C(1)–Ru(1)–P(2) | 90.1(2) | C(2)–Ru(1)–Cl(2) | 86.6(2) |
| C(2)–Ru(1)–P(2) | 91.0(2) | P(1)–Ru(1)–Cl(2) | 84.49(7) |
| P(2)–Ru(1)–P(1) | 173.05(7) | P(2)–Ru(1)–Cl(2) | 89.63(6) |
| C(1)–Ru(1)–Cl(1) | 92.9(3) | Cl(1)–Ru(1)–Cl(2) | 89.91(7) |
| C(2)–Ru(1)–Cl(1) | 176.5(2) | ||
| 6·0.5CH2Cl2a | |||
| Ru(1)–C(1) | 1.880(9) | Ru(1)–Cl(1) | 2.432(2) |
| Ru(1)–P(1) | 2.410(1) | ||
| C(1)–Ru(1)–C(1)#1 | 89.7(5) | C(1)–Ru(1)–Cl(1) | 178.6(2) |
| C(1)–Ru(1)–P(1) | 94.1(2) | P(1)–Ru(1)–Cl(1)#1 | 87.24(5) |
| C(1)–Ru(1)–P(1)#1 | 91.3(2) | P(1)–Ru(1)–Cl(1) | 87.34(5) |
| P(1)#1–Ru(1)–P(1) | 172.4(1) | Cl(1)#1–Ru(1)–Cl(1) | 89.4(1) |
| C(1)–Ru(1)–Cl(1)#1 | 90.5(2) | ||
N-(Diphenylphosphinomethyl)morpholine, Ph2PCH2morph, is a non-rigid ligand whose phosphorus and nitrogen atoms are more basic than those in Ph2Ppy. On the other hand, the basicity of Ru is higher than that of Fe.4b This may be the reason that Ph2PCH2morph reacts with Na[RuH(CO)4] to give a higher yield of compound 2 than the reaction of Ph2Ppy. Similar to the iron analog, compound 2 exhibits a strong ν(CO) at 1885 cm−1, indicating that the three CO groups are equivalent; the 31P NMR signal of 2 is a singlet at 16.8 ppm, indicating that the two phosphine moieties are chemically equivalent.
Treatment of 2 with HgI2 and CdI2 gave the corresponding hetero-bimetallic complexes 4 and 5, respectively. The IR spectra of 4 and 5 also show splitting and a shift to higher wavenumbers, as expected. The 31P NMR spectra of compounds 4 and 5 each exhibits a singlet with two low-intensity satellite peaks [2J(199Hg, 31P)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 Hz and 2J(111Cd, 31P)
123 Hz and 2J(111Cd, 31P)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 Hz]. The coupling values are comparable with those in complexes containing Ru–Hg and Ru–Cd bonds, for example: 432 Hz17a in cis-Ru(CO)3(μ-Ph2Ppy)(HgBr)2, 109.9 Hz4b in [trans-Ru(CO)3(μ-Ph2Ppy)2HgCl]2(Hg2Cl6)
and 23 Hz4b in trans-Ru(CO)3(μ-Ph2Ppy)2Cd(ClO4)2. Our attempt to prepare a Ru–Hg complex by treating 2 with HgCl2 was unsuccessful and compound 6 of formula trans, cis-Ru(CO)2(μ-Ph2PCH2morph)2Cl2 was isolated. Compound 6 was also obtained from a solution of 6 in dichloromethane or chloroform upon prolonged standing. Single crystals of the solvates 6·CH2Cl2 and 6·0.5CH2Cl2 were obtained from crystallization of 6 from CH2Cl2–Et2O and CH2Cl2–MeOH, respectively.
28 Hz]. The coupling values are comparable with those in complexes containing Ru–Hg and Ru–Cd bonds, for example: 432 Hz17a in cis-Ru(CO)3(μ-Ph2Ppy)(HgBr)2, 109.9 Hz4b in [trans-Ru(CO)3(μ-Ph2Ppy)2HgCl]2(Hg2Cl6)
and 23 Hz4b in trans-Ru(CO)3(μ-Ph2Ppy)2Cd(ClO4)2. Our attempt to prepare a Ru–Hg complex by treating 2 with HgCl2 was unsuccessful and compound 6 of formula trans, cis-Ru(CO)2(μ-Ph2PCH2morph)2Cl2 was isolated. Compound 6 was also obtained from a solution of 6 in dichloromethane or chloroform upon prolonged standing. Single crystals of the solvates 6·CH2Cl2 and 6·0.5CH2Cl2 were obtained from crystallization of 6 from CH2Cl2–Et2O and CH2Cl2–MeOH, respectively.
ORTEP drawings with atom numbering for molecules 4–6 in 4·Et2O, 5·Et2O, and 6·CH2Cl2 are shown in Figs. 2–4, respectively. Selected bond lengths and angles are listed in Table 2. All these complexes display a distorted octahedral geometry about the Ru atom, in which the RuP2 unit is nearly linear with a P–Ru–P angle close to 180°. The three CO ligands and M in 4 and 5, as well as the two CO moieties and two Cl atoms in 6, lie in a plane perpendicular to the RuP2 axis.
![Perspective view (35% thermal ellipsoids) of the molecular structure of [Ru(CO)3(μ-Ph2PCH2morph)2HgI2]
[Ph2PCH2morph = N-(diphenylphosphinomethyl)morpholine], 4, in 4·Et2O.](/image/article/2002/NJ/b108287f/b108287f-f2.gif) | ||
Fig. 2 Perspective view (35% thermal ellipsoids) of the molecular structure of [Ru(CO)3(μ-Ph2PCH2morph)2HgI2]
[Ph2PCH2morph![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N-(diphenylphosphinomethyl)morpholine], 4, in 4·Et2O. N-(diphenylphosphinomethyl)morpholine], 4, in 4·Et2O. | ||
![Perspective view (35% thermal ellipsoids) of the molecular structure of [Ru(CO)3(μ-Ph2PCH2morph)2CdI2], 5, in 5·Et2O.](/image/article/2002/NJ/b108287f/b108287f-f3.gif) | ||
| Fig. 3 Perspective view (35% thermal ellipsoids) of the molecular structure of [Ru(CO)3(μ-Ph2PCH2morph)2CdI2], 5, in 5·Et2O. | ||
![Perspective view (35% thermal ellipsoids) of the molecular structure of trans,cis-[Ru(CO)2(Ph2PCH2morph)2Cl2], 6, in 6·CH2Cl2.](/image/article/2002/NJ/b108287f/b108287f-f4.gif) | ||
| Fig. 4 Perspective view (35% thermal ellipsoids) of the molecular structure of trans,cis-[Ru(CO)2(Ph2PCH2morph)2Cl2], 6, in 6·CH2Cl2. | ||
Complexes 4 and 5 are isomorphous with their iron(0) analogs.5b In complex 4, the Hg atom is only three-coordinated with a Ru–Hg distance of 2.7075(4)
Å, which is comparable to those of the Ru–Hg dative bond in cis-Ru(CO)3(μ-Ph2Ppy)(HgBr)2
[2.628(2) and 2.602(2)
Å]17a and cis-Ru(CO)4[HgRu3(CO)9(μ-C![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) C-t-Bu)]2
[2.658(1) and 2.655(1)
Å],17b but shorter than those in [Ru6(μ-Hg)4(μ-ampy)2(CO)18]
[2.839(1), 2.859(1) and 2.841(1)
Å,
ampy
C-t-Bu)]2
[2.658(1) and 2.655(1)
Å],17b but shorter than those in [Ru6(μ-Hg)4(μ-ampy)2(CO)18]
[2.839(1), 2.859(1) and 2.841(1)
Å,
ampy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-amino-6-methyl-pyridine].17c The relatively long Hg(1)⋯N(1) and Hg(1)⋯N(2) distances of 2.790 and 3.789 Å imply that only one nitrogen atom has a weak interaction with the mercury atom (the distance of 2.790 Å is longer than the range of 2.26–2.56 Å found for mercury–tertiary amine complexes.)18
2-amino-6-methyl-pyridine].17c The relatively long Hg(1)⋯N(1) and Hg(1)⋯N(2) distances of 2.790 and 3.789 Å imply that only one nitrogen atom has a weak interaction with the mercury atom (the distance of 2.790 Å is longer than the range of 2.26–2.56 Å found for mercury–tertiary amine complexes.)18
In complex 5, the Cd atom is four-coordinated with a Ru–Cd bond distance of 2.7750(9) Å, which is comparable to the sum of Ru and Cd metallic radii (2.73 Å) and those of the Ru(0)–Cd(II) dative bond in trans-Ru(CO)3(μ-Ph2Ppy)2CdCl2 [2.771(1) Å] and trans-Ru(CO)3(μ-Ph2Ppy)2Cd(ClO4)2 [2.705(1) Å].4b This is suggestive of a strong Ru-Cd bond in compound 5. The Cd–N distances are 2.519(5) and 4.0 Å, indicating that only one nitrogen atom is coordinated to the cadmium atom.
The 1 : 1 and 2 : 1 solvates of compound 6 with CH2Cl2 crystallize in different space groups: triclinic (P1) and tetragonal (I4t/a), respectively. Molecule 6 in 6·CH2Cl2 has pseudo-Cs symmetry in which the two phosphine ligands are almost mirror images across the plane containing the Ru atom, two CO groups and two cis chloro ligands. In the crystal structure of 6·0.5CH2Cl2, a crystallographic C2 axis bisects the planar Ru(CO)2Cl2 unit of the molecule.
Conclusion
We have successfully developed a convenient new procedure for the preparation of trans-Ru(CO)3(L)2 (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphine bearing a N-donor substituent) and used the tridentate organometallic ligands 1
[L
phosphine bearing a N-donor substituent) and used the tridentate organometallic ligands 1
[L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-(diphenylphoshino)pyridine] and 2
[L
2-(diphenylphoshino)pyridine] and 2
[L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N-(diphenylphosphinomethyl)morpholine] to react with the Lewis acids AgCF3SO3, HgI2 and CdI2 to form hetero-binuclear complexes containing a Ru0→Mn+
[M
N-(diphenylphosphinomethyl)morpholine] to react with the Lewis acids AgCF3SO3, HgI2 and CdI2 to form hetero-binuclear complexes containing a Ru0→Mn+
[M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag(I), Hg(II), Cd(II)] dative bond.
Ag(I), Hg(II), Cd(II)] dative bond.
Acknowledgements
This work was supported by Hong Kong Research Grants Council Earmarked Grant ref. no. CUHK 4022/98P.References
- See, for example: (a) J. P. Farr, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 1980, 102, 6654 CrossRef CAS; (b) A. L. Balch, Prog. Inorg. Chem., 1993, 41, 239 Search PubMed.and references therein; (c) C. Franciò, R. Scopelliti, C. G. Arena, G. Bruno, D. Drommi and F. Faraone, Organometallics, 1998, 17, 338 CrossRef CAS; (d) K. B. Shiu, S. S. Young, S. I. Chen, J. Y. Chen, H. J. Wang, S. L. Wang, S. M. Peng and Y. H. Liu, Organometallics, 1999, 18, 4244 CrossRef CAS; (e) T. Yamaguchi, F. Yamazaki and T. Ito, J. Am. Chem. Soc., 1999, 121, 7405 CrossRef CAS; (f) T. Murahashi, T. Otani, T. Okuno and H. Kurosawa, Angew. Chem., Int. Ed., 2000, 39, 537 CrossRef CAS; (g) F. A. Cotton, E. V. Dikarev, M. A. Petrukhina and R. E. Taylor, J. Am. Chem. Soc., 2001, 123, 5831 CrossRef CAS; (h) C. Floriani, E. Solari, F. Franceschi, R. Scopelliti, P. Belanzoni and M. Rosi, Chem. Eur. J., 2001, 7, 3052 CrossRef CAS; (i) P. Braunstein, J. Durand, M. Knorr and C. Strohmann, Chem. Commun., 2001, 211 RSC; (j) P. Braunstein, M. Veith, J. Blin and V. Huch, Organometallics, 2001, 20, 627 CrossRef CAS; (k) S. M. Reid, J. T. Mague and M. J. Fink, J. Am. Chem. Soc., 2001, 123, 4081 CrossRef CAS; (l) A. F. Heyduk and D. G. Nocera, Science, 2001, 293, 1639 CrossRef CAS.
- For example: (a) R. J. Batchelor, H. B. Davis, F. W. B. Einstein and R. K. Pomeroy, J. Am. Chem. Soc., 1990, 112, 2036 CrossRef CAS; (b) J. L. Male, H. B. Davis, R. K. Pomeroy and D. R. Tyler, J. Am. Chem. Soc., 1994, 116, 9353 CrossRef CAS.
- (a) G. R. Newkome and D. C. Hager, J. Org. Chem., 1978, 43, 947 CrossRef; (b) G. R. Newkome, Chem. Rev., 1993, 93, 2067 CrossRef CAS; (c) Z.-Z. Zhang and H. Cheng, Coord. Chem. Rev., 1996, 147, 1 CrossRef; (d) P. Espinet and K. Soulantica, Coord. Chem. Rev., 1999, 195, 499 CrossRef; (e) V. J. Catalano, B. L. Bennett, S. Muratidis and B. C. Noll, J. Am. Chem. Soc., 2001, 123, 173 CrossRef CAS; (f) S. Gladiali, L. Pinna, C. G. Arena, E. Rotondo and F. Faraone, J. Mol. Catal., 1991, 66, 183 CrossRef CAS; (g) E. Drent, P. Arnoldy and P. H. M. Budzelaar, J. Organomet. Chem., 1994, 475, 57 CrossRef CAS; (h) A. Scrivanti and U. Matteoli, Tetrahedron Lett., 1995, 36, 9015 CrossRef CAS; (i) Z.-Z. Zhang, H.-P. Xi, W.-J. Zhao, K.-Y. Jiang, R.-J. Wang, H.-G. Wang and Y. Wu, J. Organomet. Chem., 1993, 454, 221 CrossRef CAS.
- For previous syntheses of this compound, see: (a) A. Maisonnet, J. P. Farr, M. M. Olmstead, C. T. Hunt and A. L. Balch, Inorg. Chem., 1982, 21, 3961 CrossRef CAS; (b) W.-H. Chan, Z.-Z. Zhang, T. C. W. Mak and C.-M. Che, J. Chem. Soc., Dalton Trans., 1998, 803 RSC.
- (a) S.-M. Kuang, Z.-Z. Zhang, Q.-G. Wang and T. C. W. Mak, Inorg. Chem., 1998, 37, 6090 CrossRef CAS; (b) H.-B. Song, Q.-M. Wang, Z.-Z. Zhang and T. C. W. Mak, J. Organomet. Chem., 2000, 605, 15 CrossRef CAS.
- Bruker SMART 5.0 and SAINT 4.0 for Windows NT, Area Detector Control and Integration Software, Bruker Analytical X-Ray Systems, Inc., Madison, WI, USA, 1998..
- G. M. Sheldrick, SADABS, Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Germany, 1996..
- T. Higashi, ABSCOR, An Empirical Absorption Correction Based on Fourier Coefficient Fitting, Rugaku Corporation, Tokyo, Japan, 1995..
- G. M. Sheldrick, SHELXTL 5.10 for Windows NT, Structure Determination Software Programs, Bruker Analytical X-Ray Systems, Inc., Madison, WI, USA, 1997..
- (a) N. Ahmad, J. J. Levison, S. D. Robinson and M. F. Uttley, Inorg. Synth., 1974, 15, 45 CAS; (b) A. J. P. Domigos, J. A. S. Howell, B. F. G. Johson and J. Lewis, Inorg. Synth., 1976, 16, 103; (c) A. J. P. Domigos, J. A. S. Howell, B. F. G. Johson and J. Lewis, Inorg. Synth., 1990, 28, 52.
- (a) R. L. Keiter, E. A. Keiter, K. H. Hecker and C. A. Boecker, Organometallics, 1988, 7, 2466 CrossRef CAS; (b) R. L. Keiter, E. A. Keiter, C. A. Boecker, D. R. Micher and D. R. Hecker, Inorg. Synth., 1997, 31, 210 CAS; (c) J. J. Brunet, F. B. Kindela, D. Labroue and D. Neibecher, J. Organomet. Chem., 1989, 368, 209 CrossRef CAS; (d) J. J. Brunet, G. Commenges, F. B. Kindela and D. Neibecker, Organometallics, 1992, 11, 1343 CrossRef CAS; (e) J. J. Brunet, F. B. Kindela and D. Neibecker, Inorg. Synth., 1992, 29, 151 CAS; (f) J. J. Brunet, R. Chauvin, O. Diallo, F. Kindela, P. Leglaye and D. Neibecker, Coord. Chem. Rev., 1998, 180, 331 CrossRef.
- (a) J. D. Cotlon, M. I. Bruce and F. G. A. Stone, J. Chem. Soc. A, 1968, 2162 RSC; (b) J. P. Collman, D. W. Murphy, E. B. Fleischer and D. Swift, Inorg. Chem., 1974, 13, 1 CrossRef CAS.
- J. H. MacNeil, A. C. Chiverton, S. Fortier, M. C. Baird, R. C. Hynes, A. J. Williams, K. F. Preston and T. Ziegler, J. Am. Chem. Soc., 1991, 113, 9384 CrossRef.
- L. Song and W. C. Trogler, Angew. Chem., Int. Ed. Engl., 1992, 31, 770 CrossRef.
- (a) S.-M. Kuang, H. Cheng, L.-J. Sun, Z.-Z. Zhang, Z.-Y. Zhou, B.-M. Wu and T. C. W. Mak, Polyhedron, 1996, 15, 3417 CrossRef CAS; (b) S.-L. Li, T. C. W. Mak and Z.-Z. Zhang, J. Chem. Soc., Dalton Trans., 1996, 3475 RSC; (c) S.-M. Kuang, Z.-Z. Zhang, B.-M. Wu, T. C. W. Mak and Z.-Z. Zhang, J. Organomet. Chem., 1997, 540, 55 CrossRef CAS; (d) H.-B. Song, Z.-Z. Zhang and T. C. W. Mak, Inorg. Chem., 2001, 40, 5928 CrossRef CAS; (e) D.-J. Cui, Q.-S. Li, F.-B. Xu, X.-B. Leng and Z.-Z. Zhang, Organometallics, 2001, 20, 4126 CrossRef CAS.
- (a) M. J. Freeman, M. Green, A. G. Orpen, I. D. Salter and F. G. A. Stone, J. Chem. Soc., Chem. Commun., 1983, 1332 RSC; (b) J. A. Ladd, H. Hope and A. L. Balch, Organometallics, 1984, 3, 1838 CrossRef CAS; (c) M. I. Bruce, M. L. Williams, J. M. Patrick, B. W. Skelton and A. H. White, J. Chem. Soc., Dalton Trans., 1986, 2557 RSC.
- (a) S.-M. Kuang, F. Xue, Z.-Y. Zhang, T. C. W. Mak and Z.-Z. Zhang, J. Organomet. Chem., 1998, 559, 31 CrossRef CAS; (b) E. Rosenberg, D. Ryckman, I.-N. Hsu and R. W. Gellert, Inorg. Chem., 1986, 25, 194 CrossRef CAS; (c) P. L. Andreu, J. A. Cabeza, A. Llamazares and V. Riera, J. Organomet. Chem., 1991, 420, 431 CrossRef CAS.
- See, for example: (a) C. M. Hughes, M. C. Favas, B. W. Skelton and A. H. White, Aust. J. Chem., 1985, 38, 1521 CAS; (b) C. A. Ghilardi, S. Midollini, A. Orlandini and A. Vacca, J. Organomet. Chem., 1994, 471, 29 CrossRef CAS; (c) D. C. Bebout, D. E. Ehmann, J. C. Trinidad and K. K. Crahan, Inorg. Chem., 1997, 36, 4257 CrossRef CAS; (d) F. Cecconi, C. A. Ghilardi, S. Midollini and A. Orlandin, Inorg. Chim. Acta, 1998, 269, 274 CrossRef CAS; (e) A. Albinati, V. Gramlich, G. Anderegg and F. Lianza, Inorg. Chim. Acta, 1998, 274, 219 CrossRef CAS; (f) D. C. Bebout, J. F. Bush II and K. C. Crahan, Inorg. Chem., 1998, 37, 4641 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2002 |


