“Scission–template–transportation” route to controllably synthesize CdIn2S4 nanorods
Jun Lu, Yi Xie*, Guoan Du, Xuchuan Jiang, Liying Zhu, Xingjun Wang and Yitai Qian
Structure Research Lab. and Lab. of Nanochemistry & Nanomaterials, University of Science & Technology of China, Hefei, Anhui 230026, P. R. China. E-mail: yxie@ustc.edu.cn
First published on 15th November 2001
Abstract
By using a novel method denoted the “Scission–template–transportation” route, CdIn2S4 nanorods with stoichiometric composition and high quality can be prepared in an ethylenediamine–ethanol mixed solvent at a relatively low temperature. Transmission electron microscopy images showed that the product from the 1∶1 mixed solvent was rod-like with an average size of 25 × 700 nm. Ethylenediamine serves as the nucleophile for the formation of the inorganic [Cd2S2] core by scission of the thione groups of cadmium bis(diethyldithiocarbanate) [Cd(DDTC)2]2 while ethanol acts as the transportation reagent for InCl3 in the process. These factors are both conducive to the growth of CdIn2S4 nanorods. The reaction proceeds through one-dimensional [CdS]n clusters, which act as intermediate templates for the subsequent growth of CdIn2S4 nanorods. Through adjusting the ethylenediamine∶ethanol ratio, the size of the CdIn2S4 nanorods can be easily controlled. The products were also investigated by X-ray powder diffraction, X-ray photoelectron spectroscopy and ICP elemental analysis.
Introduction
Since the discovery of carbon nanotubes in 1991,1 the fabrication or synthesis of one-dimensional nanometer materials has been the focus of much attention because of their special properties.2 Compared to micrometer-diameter whiskers, these fascinating systems are expected to exhibit remarkable mechanical properties, as well as electrical, optical, and magnetic properties different from those of the corresponding bulk materials.3 These new nanoscale materials have potential applications in both mesoscopic research and the development of nanodevices. Thus, developing controlled synthesis methods is always one of the most important goals of materials scientists.So far, a series of binary compounds with 1D nanostructure such as WS2,4 MoS2,5 carbides,6 GaN,7 Si3N4,8 and β-SiC9 have been prepared successfully, and several significant synthesis methods for nanorods have also been developed. By using carbon nanotubes as the template, Dai et al.6 first succeeded in synthesizing carbide nanorods. This involves preparing the materials on or within a pre-existing template of the desired size and shape of the final product, i.e., template-mediated growth. Meng et al.9 developed a method to prepare a “β-SiC nanorod within a SiO2 nanorod”, 1D composite nanostructure. However, all of these reported methods require high processing temperatures and the yields are relatively low; furthermore, they usually focus on the binary compounds.
In recent years, multinary chalcogenide compounds have been the subject of intense research.10 CdIn2S4 is a ternary semiconductor compound crystallizing in the spinel structure. Its optical and electrical properties have been intensively investigated in the past. The results demonstrate peculiar characteristics due to the presence of native defects, the configuration and concentration of which strongly depend on crystal growth conditions which may be varied by subsequent thermal treatments.11 Considerable progress has been made in the synthesis of ternary semiconductor crystallites.12,13 However, these traditional solid-state reaction methods customarily require elevated processing temperatures (800–1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C), long reaction times, and special apparatus; furthermore, vacancies or interstitial defects in the chalcopyrite lattice cause these
compounds to have poor crystal quality and poor optical transparency. It is well known that the resulting physical properties and microstructure of materials rely highly on the different synthesis methods. Recently, some fascinating materials have been prepared by using various solution-chemical synthesis techniques. Our group have prepared CuInSe2 nanowhiskers14 and AgInS2 nanoparticles15 by a solvothermal route. These chemical methods make it easier to control the particle sizes and their distribution. However, it is more difficult to control the morphology of the products.
°C), long reaction times, and special apparatus; furthermore, vacancies or interstitial defects in the chalcopyrite lattice cause these
compounds to have poor crystal quality and poor optical transparency. It is well known that the resulting physical properties and microstructure of materials rely highly on the different synthesis methods. Recently, some fascinating materials have been prepared by using various solution-chemical synthesis techniques. Our group have prepared CuInSe2 nanowhiskers14 and AgInS2 nanoparticles15 by a solvothermal route. These chemical methods make it easier to control the particle sizes and their distribution. However, it is more difficult to control the morphology of the products.
Herein, we report a novel strategy for controllably synthesizing CdIn2S4 nanorods by a solvothermal technique at a relatively low temperature of 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. In our previous work,16 we first reported that quantum-confined CdS nanowires can be prepared from cadmium bis(diethyldithiocarbanate)
([Cd(DDTC)2]2) by removal of the four thione groups with ethylenediamine (en). The CdS nanowires obtained from this route are relatively uniform because the reaction takes place homogeneously, and the yield of nanowires is very high (>90% from the raw materials). In this work, we successfully extended this method to controllably synthesize ternary chalcogenide CdIn2S4 nanorods. In the approach, cadmium bis(diethyldithiocarbanate), which can be regarded as an inorganic core [Cd2S2] with
four capping groups, and indium chloride (InCl3) were used as the reactants. The reaction was carried out in a mixed solvent of ethanol and ethylenediamine (en) in an autoclave. Ethylenediamine is crucial in the process as this nucleophile causes scission of the four capping groups from cadmium bis(diethyldithiocarbanate), with the newly-formed [Cd2S2] inorganic cores released form cadmium bis(diethyldithiocarbanate) assembling into one-dimensional [CdS]n clusters which may act as intermediate templates for the growth of CdIn2S4 nanorods. Since indium chloride is soluble in the ethanol cosolvent it can be easily transported to the surface of the intermediate template. We thus coin the term “Scission–template–transportation” for this route. The possible reaction mechanism can be represented as shown in Scheme 1.
°C. In our previous work,16 we first reported that quantum-confined CdS nanowires can be prepared from cadmium bis(diethyldithiocarbanate)
([Cd(DDTC)2]2) by removal of the four thione groups with ethylenediamine (en). The CdS nanowires obtained from this route are relatively uniform because the reaction takes place homogeneously, and the yield of nanowires is very high (>90% from the raw materials). In this work, we successfully extended this method to controllably synthesize ternary chalcogenide CdIn2S4 nanorods. In the approach, cadmium bis(diethyldithiocarbanate), which can be regarded as an inorganic core [Cd2S2] with
four capping groups, and indium chloride (InCl3) were used as the reactants. The reaction was carried out in a mixed solvent of ethanol and ethylenediamine (en) in an autoclave. Ethylenediamine is crucial in the process as this nucleophile causes scission of the four capping groups from cadmium bis(diethyldithiocarbanate), with the newly-formed [Cd2S2] inorganic cores released form cadmium bis(diethyldithiocarbanate) assembling into one-dimensional [CdS]n clusters which may act as intermediate templates for the growth of CdIn2S4 nanorods. Since indium chloride is soluble in the ethanol cosolvent it can be easily transported to the surface of the intermediate template. We thus coin the term “Scission–template–transportation” for this route. The possible reaction mechanism can be represented as shown in Scheme 1.
 | ||
| Scheme 1 Mechanism of formation of CdIn2S4 nanorods prepared by the novel “Scission–template–transportation” route in ethylenediamine–ethanol. | ||
Experimental
All reagents were of analytical grade while indium chloride was refluxed in thionyl chloride (SOCl2) for 45 minutes to remove the water of crystallisation. In a typical procedure, analytical grade [Cd(DDTC)2]2 (0.408 g, 0.5 mmol)17 and InCl3 (0.111 g, 0.5 mmol) were placed in a 50 mL autoclave which was filled with a 40 ml of ethylenediamine–ethanol (1∶1). The autoclave was heated and maintained at 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 8 h before allowing to cool to room temperature naturally. The products were filtered off, washed sequentially with CS2, 1 M HCl, distilled water, and absolute ethanol to remove the byproducts. Finally, the products were dried in vacuum at room temperature for 4 h.
°C for 8 h before allowing to cool to room temperature naturally. The products were filtered off, washed sequentially with CS2, 1 M HCl, distilled water, and absolute ethanol to remove the byproducts. Finally, the products were dried in vacuum at room temperature for 4 h.Powder X-ray diffraction (XRD) patterns were performed on a Japan Rigaku D/Max γA rotating anode X-ray diffractometer with Ni-filtered Cu-Kα radiation (λ = 1.54178 Å). Transmission electron microscopy (TEM) measurements were made on a Hitachi H-800 transmission electron microscope with an accelerating voltage of 200 kV. Samples were deposited from ethanol solutions of the products on to thin amorphous carbon films supported by copper grids. The purity and composition of the products were detected by X-ray photoelectron spectra (XPS) on an ECSALab MKII instrument with Mg-Kα radiation as the exciting source. The binding energies obtained in the XPS analysis were corrected by referencing the C 1s peak to 284.60 eV. Atomic ratios in samples were measured using inductively coupled plasma (ICP) spectroscopy with a Seiko Electronics SPD 1200A ICP emission analyzer with a pump flow of 1.85 ml min−1 and a flow rate of auxiliary gas (Ar 99.99%) of 0.5 l min−1. Samples were subsequently dissolved in heated dilute HNO3 solution for further elemental analysis.
Results and discussion
The reaction of cadmium bis(diethyldithiocarbanate) [Cd(DDTC)2]2 and indium chloride in ethylenediamine–ethanol mixed solvent at 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C resulted in an orange red precipitate with the XRD pattern of the sample shown in Fig. 1. All the peaks could be indexed to the cubic CdIn2S4 phase with cell constant a = 10.867 Å, in agreement with the reported data in the literature.18 Using the Scherrer formula19 for the (hkl) peaks (111), (311), (511), (531) and (731), the average diameter of the nanorods is estimated as 30 nm.
°C resulted in an orange red precipitate with the XRD pattern of the sample shown in Fig. 1. All the peaks could be indexed to the cubic CdIn2S4 phase with cell constant a = 10.867 Å, in agreement with the reported data in the literature.18 Using the Scherrer formula19 for the (hkl) peaks (111), (311), (511), (531) and (731), the average diameter of the nanorods is estimated as 30 nm. | ||
| Fig. 1 XRD pattern of the as-prepared product from ethylenediamine–ethanol (1∶1). | ||
X-Ray photoelectron spectroscopy (XPS) has been used to measure the elemental composition of the as-prepared nanocrystals and examine oxidation of the nanocrystal surface. A survey spectrum is shown in Fig. 2A. The survey indicates the presence of Cd, In and S as well as C from the reference and O from absorbed gaseous molecules. Otherwise the spectrum is clean with absence of peaks for Cl or N from the reactants.
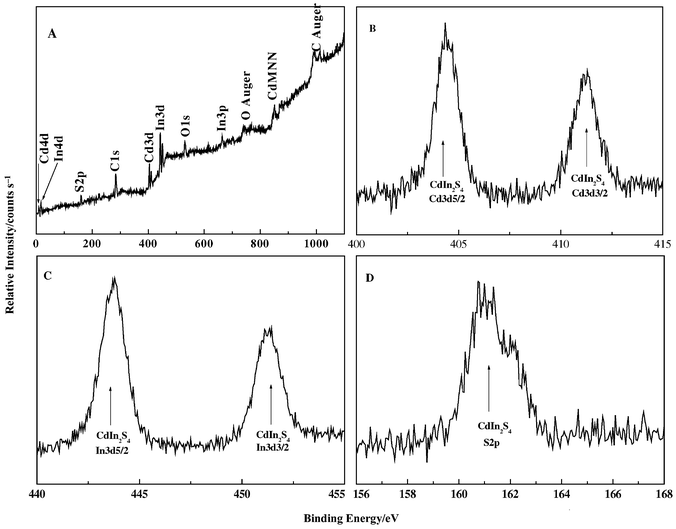 | ||
| Fig. 2 XPS spectra for CdIn2S4 nanorods, A: XPS survey spectra for the product; B: high-resolution XPS scans of Cd 3d cores for the CdIn2S4 nanorods; C: high-resolution XPS scans of In 3d cores for CdIn2S4 nanorods; D: high-resolution XPS scans of S 2p cores for CdIn2S4 nanorods. The Cd 3d core is spin–orbit spilt into 3d5/2 and 3d3/2 levels with the 3d5/2 peak at 404.4 eV. The In 3d core is also spin–orbit spilt into 3d5/2 and 3d3/2 levels with the 3d5/2 peak at 443.8 eV. | ||
Higher resolution spectra were also taken of the Cd 3d, In 3d and S 2p regions. The Cd 3d core is spin–orbit spilt into 3d5/2 and 3d3/2 levels with the 3d5/2 peak at 404.4 eV (Fig. 2B). This result indicates that there is no CdS in the final product since the cadmium core in CdS with a 3d5/2 peak at 405.3 eV is not evident.20 The In 3d core is also spin–orbit spilt into In 3d5/2 and In 3d3/2 levels with the 3d5/2 peak at 443.8 eV (Fig. 2C).The S core region shows one peak at 160.9 eV (Fig. 2D). These results are all in accord with the literature.20 The peak areas of these high-resolution scans were measured and used to calculate the elemental ratio for the nanocrystals. The quantification of peaks gives the ratio of Cd∶In∶S as 1∶1.985∶3.991, which closely agrees with that obtained from EDX analysis. These results are also nearly coincident with those obtained from ICP elemental analysis (CdIn1.992S3.986). As a result of our XPS, EDX and ICP elemental analyses, we conclude that the nanocrystals are close to stoichiometric CdIn2S4.
The structure and morphology of the sample was examined by transmission electron microscopy (TEM) and a typical TEM image is shown in Fig. 3A. The CdIn2S4 crystals appeared to display rod-like morphology with diameters of 20–25 nm and lengths of 600–750 nm. The electron diffraction (ED) pattern shown in Fig. 3B was obtained from a selected area of CdIn2S4 nanorods shown in Fig. 3A with a convergent beam, and revealed that the CdIn2S4 nanorods were single crystals.
 | ||
| Fig. 3 TEM image (A) and the electron diffraction pattern (B) of a CdIn2S4 nanorod sample prepared from ethylenediamine–ethanol (1∶1). | ||
In the processes of preparing CdIn2S4 nanorods, selecting ethylenediamine–ethanol as the mixed solvent fulfills an important role for the controlled synthesis of CdIn2S4 nanorods. Removal of the four capping groups of cadmium bis(diethyldithiocarbanate) by ethylenediamine leads to the release of inorganic [Cd2S2] cores from [Cd(DDTC)2]2, which can assemble in to one-dimensional [CdS]n clusters, which act as intermediate templates for the subsequent growth of CdIn2S4 nanorods. We discovered that ethylenediamine is the most suitable nucleophilic solvent for the formation of CdIn2S4 nanorods in the experiments. If other nucleophiles such as pyridine and diethylamine were used to remove the capping groups, no rod-like products were obtained. If only ethanol was used as the solvent, no CdIn2S4 was obtained since ethanol cannot act as a nucleophile to remove thione groups from cadmium bis(diethyldithiocarbanate). Ethylenediamine thus plays a critical role in the formation of rod structures and may serve as a director for the growth of the intermediate one-dimensional [CdS]n cluster templates. In recent years, ethylenediamine has been extensively used as a template in solvothermal processes. For example, Li et al. reported that the elemental reaction of Cd and S in ethylenediamine resulted in CdS nanorods,21 and they also successfully synthesized Mg(OH)2 nanorods in ethylenediamine.22 In our case, ethanol also plays an important role. Since indium chloride dissolves in ethanol, it can be easily transported to the surface of newly-formed one-dimensional [CdS]n cluster which is beneficial for the growth of CdIn2S4 nanorods. By contrast, indium chloride undergoes hydrolysis in water, which would hinder the transportation of indium chloride to the surface of CdS. When using water–ethylenediamine as the mixed solvent, only CdS nanowires rather than CdIn2S4 were obtained.
The growth process of CdIn2S4 nanorods can be regarded as consisting of two steps. The first is the formation of the intermediate one-dimensional [CdS]n cluster templates, while the second is the formation of CdIn2S4 nanorods through the reaction of indium chloride with [CdS]n clusters. Since these [CdS]n clusters have a high surface energy, and indium chloride can be transported to the cluster surfaces by ethanol, the second reaction will readily occur under the used reaction conditions. According to the above discussion, we propose a possible growth mechanism of CdIn2S4 nanorods as follows:
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Cd(S2CNEt2)2]2 + 4n [Cd(S2CNEt2)2]2 + 4n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) en → 4n en → 4n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SC(NH)2(CH2)2 + 4n SC(NH)2(CH2)2 + 4n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2NH + 2n Et2NH + 2n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2S + [CdS]n H2S + [CdS]n | (1) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CdS]n + 2n [CdS]n + 2n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) InCl3 → n InCl3 → n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CdIn2S4 CdIn2S4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (nanorods) + 3n (nanorods) + 3n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CdCl2 CdCl2 | (2) |
The second step of the reaction as conducted is a liquid–solid (LS) heterogeneous process which provides the possibility of significantly reducing the reaction temperature and annealing of the product in comparison with the traditional solid–solid (SS) heterogeneous reaction,23 which can be also proved by the following experiment. When we used benzene–ethylenediamine as the mixed solvent or only used ethylenediamine as the solvent, because of the insolubility of indium chloride in benzene or ethylenediamine (so the second step becomes a solid–solid heterogeneous process under such conditions), the reaction of CdS and InCl3 cannot take place at the applied temperature, and formation of CdIn2S4 nanorods does not occur.
In our process, the size of the products can be effectively controlled by changing the volume ratio of added solvents. When using ethanol and ethylenediamine in ratios of 1∶3, 1∶1 or 3∶1, the final product sizes examined from TEM images (Fig. 4) were 18 × 300 nm, 25 × 700 nm and 40 × 1000 nm, respectively. This influence is mainly related to the different viscosities of the reaction systems. During the formation of the final product, the competition between nanorod growth and nucleation is partially controlled by diffusion. For a high-viscosity solution, the rate of diffusion is low and CdIn2S4 can form new nuclei before migration to the surface of nuclei already present. The time of nucleation and length of the growth period of nuclei in different solution systems are not the same, giving nanorods with different sizes at the end of the growth process. An increase of ethylenediamine content, which has higher viscosity than ethanol, increases the viscosity of the reaction system. Therefore, the product size decreases with increasing of the amount of added ethylenediamine. However, the ratio of EtOH∶en must not be lower than 1∶4, otherwise the reaction will not occur.
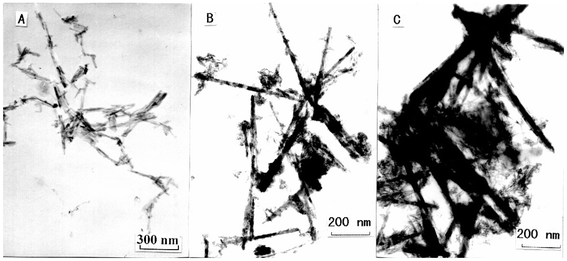 | ||
| Fig. 4 TEM images for CdIn2S4 nanorods prepared in mixed solvents with different EtOH∶en ratios: 1∶3 (A), 1∶1 (B) and 3∶1 (C). | ||
Conclusion
In conclusion, CdIn2S4 nanorods with stoichiometric composition and high quality can be prepared by a novel method which we term “Scission–template–transportation” in an ethylenediamine–ethanol mixed solvent at a relatively low temperature. To our knowledge, this is the first report for the preparation of CdIn2S4 nanorods. In the process, ethylenediamine serves as a nucleophile for the formation of inorganic [Cd2S2] cores via scission of the thione groups from cadmium bis(diethyldithiocarbanate), and ethanol acts as the transportation reagent for InCl3. These are both conducive to the growth of CdIn2S4 nanorods. Through adjusting the ethylenediamine–ethanol ratio, we can easily control the size of the CdIn2S4 nanorods. This method can easily be controlled and is expected to be applicable to fabricate other ternary compound nanorods. The ternary CdGa2S4 nanorods have also been prepared using a similar method. Further research is underway to study the properties of these materials.Acknowledgements
Financial support from the Chinese National Foundation of Natural Science Research and Chinese Ministry of Education is gratefully acknowledged.References
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- A. M. Rao, E. Richter, S. Bandow, B. Chase, P. C. Eklund, K. A. Williams, S. Fang, K. S. Subbaswamy, M. Menon, A. Thess, R. E. Smalley, G. Dresselhaus and M. S. Dresselhaus, Science, 1997, 275, 187 CrossRef CAS.
- A. P. Alivisatos, Science, 1996, 271, 933 CrossRef CAS.
- R. Tenne, L. Margulis, M. Genut and G. Hodes, Nature, 1992, 360, 444 CrossRef CAS.
- Y. Feldman, E. Wasserman, D. J. Srolovitz and R. Tenne, Science, 1995, 267, 222 CrossRef CAS.
- H. Dai, E. W. Wong, Y. Z. Lu, S. S. Fan and C. M. Lieber, Nature, 1995, 375, 769 CrossRef CAS.
- W. Q. Han, S. S. Fan, Q. Q. Li and Y. D. Hu, Science, 1997, 277, 1287 CrossRef CAS.
- W. Q. Han, S. S. Fan, Q. Q. Li, B. L. Gu, X. B. Zhang and D. P. Yu, Appl. Phys. Lett., 1997, 71, 2271 CrossRef CAS.
- G. W. Meng, L. D. Zhang, C. M. Mo, S. Y. Zhang, Y. Qin, S. P. Feng and H. J. Li, Solid State Commun., 1998, 106, 215 CrossRef CAS.
- (a) F. J. Disalvo, Science, 1991, 247, 649; (b) W. S. Sheldrick and M. Wachhold, Angew. Chem., Int. Ed. Engl., 1997, 36, 206 CrossRef CAS; (c) C. L. Bowes and G. A. Ozin, Adv. Mater., 1996, 8, 13 CAS; (d) G. A. Ozin, Adv. Mater., 1992, 4, 612 CrossRef CAS.
- O. V. Kulikova, L. L. Kulyuk, S. I. Radautsan, S. A. Ratseev, E. E. Strumban, V. E. Tezlevan and V. I. Tsitanu, Phys. Status Solidi A, 1988, 107, 373 CAS.
- B. F. Levine, Phys. Rev. B: Condens. Matter, 1973, 7, 2600 Search PubMed.
- R. K. Route, R. S. Feigelson and R. J. Raymakers, J. Cryst. Growth, 1976, 33, 239 CrossRef CAS.
- B. Li, Y. Xie, J. X. Huang and Y. T. Qian, Adv. Mater., 1999, 11, 1456 CrossRef CAS.
- J. Q. Hu, Q. Y. Lu, K. B. Tang, Y. T. Qian, G. E. Zhou and X. M. Liu, Chem. Commun., 1999, 1093 RSC.
- P. Yan, Y. Xie, Y. T. Qian and X. M. Liu, Chem. Commun., 1999, 1293 RSC.
- [Cd(DDTC2)]2 was prepared from stoichiometric amounts of an aqueous (0.1 mol L−1) solution of NaDDTC and CdCl2. The precipitate was filtered off and washed with distilled water, and dried in vacuum for 4 h..
- Joint Committee on Powder Diffraction Standards (JCPDS), File No. 27-60 for cubic CdIn2S4..
- H. P. Klug and L. E. Alexander, in X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials, Wiley, New York, 1962, p. 491 Search PubMed.
- C. D. Wagner, W. W. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg, Handbook of X-Ray Photoelectron Spectroscopy, Perkin-Elmer Corporation. Physical Electronics Division, Eden Prairie, MN, USA, 1979 Search PubMed.
- Y. D. Li, H. W. Liao, Y. Ding, Y. T. Qian, L. Yang and G. Zhou, Chem. Mater., 1998, 10, 2301 CrossRef CAS.
- Y. D. Li, M. Sui, Y. Ding, G. H. Zhang, J. Zhuang and C. Wang, Adv. Mater., 2000, 12, 818 CrossRef CAS.
- K. S. Suslick, D. A. Hammerton and R. E. Cline, J. Am. Soc. Chem., 1986, 108, 5461 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |
