Formation of highly ordered mesoporous silica materials adopting lyotropic liquid crystal mesophases
S. A. El-Safty*a and J. Evansb
aChemistry Department, Faculty of Science, Tanta University, Tanta, Egypt. E-mail: saes@dec1.tanta.eun.eg
bDepartment of Chemistry, University of Southampton, Southampton, SO17 1BJ, UK
First published on 15th November 2001
Abstract
Mesoporous silica materials have been synthesised in strongly acidic media at pH = 1.3 using high concentrations of a non-ionic surfactant (Brij 76) as a structure-directing agent. Well-defined ordered mesoporous silicas with hexagonal (HI), lamellar (L∞) and solid phase (S) structure have been prepared at room temperature according to the lyotropic liquid crystalline mesophases of Brij 76. At high temperature (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C), highly ordered cubic (Ia3d), cubic (Im3m) and 3-d hexagonal (p63/mmc) nanostructured materials have been produced. The synthesised materials were studied by powder X-ray diffraction (XRD), the Brunauer–Emmett–Teller (BET) method for nitrogen adsorption/desorption isotherms and surface area measurements. Transmission electron
microscopy (TEM), XRD and TEM patterns for all materials show well-defined long-range porous architectures. It was found that BET surface area values of the nanostructured materials are reduced upon increasing the temperature of synthesis.
°C), highly ordered cubic (Ia3d), cubic (Im3m) and 3-d hexagonal (p63/mmc) nanostructured materials have been produced. The synthesised materials were studied by powder X-ray diffraction (XRD), the Brunauer–Emmett–Teller (BET) method for nitrogen adsorption/desorption isotherms and surface area measurements. Transmission electron
microscopy (TEM), XRD and TEM patterns for all materials show well-defined long-range porous architectures. It was found that BET surface area values of the nanostructured materials are reduced upon increasing the temperature of synthesis.
Introdution
Mesoporous silica materials are generally prepared by using surfactants as a templating agents. The synthesis can be considered in terms of a self-assembly process involving electrostatic interactions between the inorganic ions in solution and charged surfactant head groups.1–5 Hydrogen bonding interactions between the neutral primary amine micelles and neutral inorganic species are also significant in the generation of mesoporous molecular sieves.6,7The dilute surfactant solutions used in the formation of ordered, mesoporous silica, such as M41-S,1,2 FSM-16,5 and HMS,6,7 limit the ability to predict the topology of the mesophases. Also only powders of micrometer dimensions (1–2 µm) are produced. The three architectures found for the M41S family of mesoporous silicas, a hexagonal phase referred to as MCM-41, a cubic phase (Ia3d) known as MCM-48, and MCM-50, an unstable lamellar phase, have been reported elsewhere.8–11 In addition, cubic (pm3n) high quality mesoporous molecular sieves designated SBA-1 have been reported.3–5,12 Generally, the structure of the resulting mesoporous solid is affected by the reaction conditions. Changing the pH of the medium leads to the transformation of the lamellar phase of silica to the hexagonal phase.13 Other factors such as temperature and surfactant concentration have been observed to affect the structure formed.14–16
High concentrations of non-ionic surfactants have been employed for the synthesis of large uniform nanoporous monolithic silicates. In such systems lyotropic liquid crystalline phases are exploited as templates to produce long-range ordered mesoporous silicates, which are independent of the structures and charge of the amphiphiles, as shown by Attard et al.17,18 The synthesis of hexagonal (HI-silica), cubic (Ia3d-silica) and lamellar (L∞-silica) silica as well as HI-aluminium silicates have also been reported.19
Our approach uses high concentrations of the non-ionic surfactant Brij 76 as a template structure-directing agent to form well-defined long range ordered mesoporous silica materials. Nanoporous monolithic silicates including hexagonal (HI), lamellar (L∞), solid phase (S), cubic (Ia3d), cubic (Im3m), and 3-d hexagonal (p63/mmc) materials are synthesised via the lyotropic liquid crystal phase of the template and at different temperatures. The high quality materials synthesised are investigated by using XRD, TEM, and BET for N2 adsorption/desorption isotherms and surface area measurements.
Experimental
Materials
Tetramethylorthosilicate (TMOS) was obtained from Fluka. Brij 76 (polyethylene (10) stearyl ether) was supplied by Sigma–Aldrich Ltd. UK.Synthesis
In all cases of syntheses of the mesoporous silica mesophases the surfactant (Brij 76) and TMOS were mixed with agitation until homogeneous. Hydrochloric acid (diluted to pH = 1.3 with deionized water) was added quickly which led to exothermic hydrolysis. The mixture was placed under gentle vacuum until it changed from a viscous liquid to a gel-like material. As-synthesized materials were collected and allowed to stand in a sealed container at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 20 h. For room-temperature syntheses the weight ratio of surfactant∶TMOS∶HCl was 1∶2∶1 for HI-SiO2,20 1.5∶2∶1 for L∞-SiO2, and 1.3∶1.5∶1 for S-SiO2. For syntheses conducted at 60
°C for 20 h. For room-temperature syntheses the weight ratio of surfactant∶TMOS∶HCl was 1∶2∶1 for HI-SiO2,20 1.5∶2∶1 for L∞-SiO2, and 1.3∶1.5∶1 for S-SiO2. For syntheses conducted at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, the weight ratio was 1.4∶2∶1 for Ia3d-SiO2, 1.52∶2∶1 for Im3m-SiO2,
and 1.62∶2∶1 for 3-d (HI-SiO2). The surfactant was removed by calcination at 450
°C, the weight ratio was 1.4∶2∶1 for Ia3d-SiO2, 1.52∶2∶1 for Im3m-SiO2,
and 1.62∶2∶1 for 3-d (HI-SiO2). The surfactant was removed by calcination at 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (3 h under nitrogen and 14 h under oxygen) for all the silica mesophase materials.
°C (3 h under nitrogen and 14 h under oxygen) for all the silica mesophase materials.Analyses
Powder X-ray diffraction (XRD) patterns were recorded on a Siemens (θ–2θ) D5000 diffractometer with monochromated Cu-Kα radiation. Nitrogen adsorption/desorption isotherms and surface area measurements were determined following the BET method at 77 K, the data being collected with a Micromeritrics GEMINI III 2375 surface area analyzer. Transmission electron microscopy (TEM) images were recorded on a JEOL FX 2000 instrument operating at an acceleration voltage of 200 kV.Results and discussion
The isotropic liquid produced during the hydrolysis of TMOS in the Brij 76/HCl mixture was due to the formation of methanol. The presence of the methanol in the mixture destroys the mesophase structure but removal of the methanol under vacuum allowed the mesophases to form. The generation of the monolithic material under highly acidic conditions (pH = 1.3) leads to a rapid poly-condensation process without loss of the long-range order of the mesoporous materials.20,21The high concentration of Brij 76 permits the pre-existence of lyotropic liquid crystalline phases and directs the formation of monolithic nanostructured materials with different mesophases through a liquid crystal template mechanism.8,9,20 The temperature and the surfactant concentration mainly affect the phases formed,22 rather the structure and charge density of the surfactant.23
Nitrogen adsorption/desorption isotherms
N2 adsorption/desorption isotherms for the calcined mesoporous materials are shown in Figs. 1 and 2. All the mesoporous materials exhibited type IV isotherms, typical of mesoporous materials with pore size less than 40 Å.24,25 A sharp inflection between relative pressure P/Po = 0.25 and 0.5 indicates capillary condensation within uniform mesophases. This inflection point depends on the pore size and the sharpness in this step signifies a uniform pore size. In addition, the initial step at low relative pressure can be extrapolated to the origin, suggesting monolayer N2 adsorption on the walls of mesopores. Hysteresis loops in the adsorption/desorption isotherms that are intermediate between type H1-, and H2-models are shown in Fig. 1.26 Such a combination of H1 and H2 hysteresis loops can be attributed to capillary condensation associated with large pore channels.27,28 | ||
| Fig. 1 Nitrogen adsorption (solid line)/desorption (dotted line) isotherms for calcined mesoporous silicate materials for (a) HI-SiO2, (b) L∞-SiO2 and (c) S-SiO2 (relative pressure is p/po, where p is the equilibrium pressure of the adsorbate and po is the saturation pressure of the adsorbate at the temperature of the adsorbent; V is the volume adsorbed at STP). | ||
 | ||
| Fig. 2 Nitrogen adsorption (solid line)/desorption (dotted line) isotherms for calcined mesoporous silicate materials for (a) Ia3d-SiO2, (b) Im3m-SiO2 and (c) P63/mmc-SiO2. | ||
On the other hand, N2 adsorption/desorption isotherms (shown in Fig. 2) show a clear type H3 hysteresis loop at higher relative pressure. This may be attributed to N2 filling the textural mesopores associated with slit-shaped or plate-like particles.29
The BET surface area and the total pore volume at relative pressure (P/Po) = 0.953 of the calcined materials were calculated and are summarised in Table 1. It seems that the concentration of the template and the temperature during the synthesis of the mesoporous materials influence both the surface area and pore volume. The surface area of the mesoporous materials was found to be in the range 580–950 m2 g−1, reflecting a high internal surface area of the mesoporous frameworks.20,21
| Mesophase material | Template concentration(%) | T/°C | V/cm3 g−1 | a/Å | A/m2 g−1 |
|---|---|---|---|---|---|
| HI-SiO2 | 50 | 25 | 0.656 | 38 | 787 |
| L∞-SiO2 | 75 | 25 | 0.765 | 37 | 852 |
| S-SiO2 | 87 | 25 | 0.861 | 39 | 950 |
| Ia3d-SiO2 | 70 | 60 | 0.582 | 36 | 624 |
| Im3m-SiO2 | 76 | 60 | 0.564 | 35 | 604 |
| P63/mmc-SiO2 | 81 | 60 | 0.511 | 34 | 580 |
The Horvath–Kawazoe (HK) pore size distribution for calcined mesoporous materials are shown in Fig. 3, and presented in Table 1. Generally, desorption data are often used for assessment of the distribution pore size curve. However, a type H3 hysteresis loop found for the high temperature synthesised mesoporous materials is unlikely to yield a reliable estimation of pore size distribution.26 Therefore, the adsorption data was used to determine the mesopore size distribution as shown in Fig. 3(d)–(f).
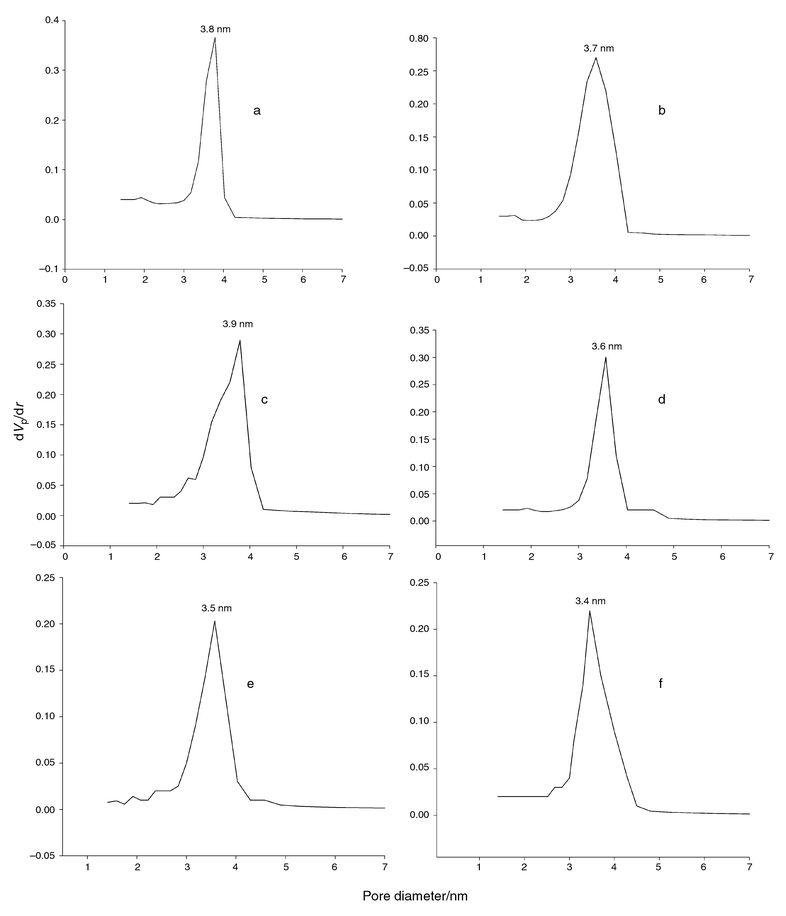 | ||
| Fig. 3 Horvath–Kawazoe plots of N2 desorption data for (a) HI-SiO2, (b) L∞-SiO2 and (c) S-SiO2 and N2 adsorption data for (d) Ia3d-SiO2, (e) Im3m-SiO2 and (f) P63/mmc-SiO2 mesoporous materials (dVp/dr is the derivative of the nitrogen volume adsorbed with respect to the pore diameter of the adsorbent). | ||
Powder X-ray diffraction (XRD)
XRD was used primarily to probe the periodicity of the mesoporous materials. These types of material display a very high intensity peak around 2θ = 2° together with low intensity peaks in the 2θ range 3–8°.1,2Figs. 4 and 5 illustrate that the calcined samples of the lyotropic liquid crystal mesophase materials yield well-resolved XRD patterns. A long-range hexagonal order of HI-SiO2 was observed, as indicated by the presence of d100, d110 and d200 reflections, Fig. 4(a).30–32 The low-angle XRD pattern of HI-SiO2 shows a d100-spacing of 48 Å, which is similar to that reported for lyotropic mesophase silicate materials.33 The pattern shown in Fig. 4(b) can be indexed to the lamellar spacing ratios 1∶2∶3. The presence of these hkl reflections with a unit cell parameter of 44 Å for the synthesised mesoporous sample with 75% of Brij 76 at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C has been established for lamellar phase materials.34 By increasing of the template concentration to 87%
(w/w) of Brij 76 (Table 1), the solid phase mesoporous material (S-SiO2) can be characterised in accord with the Brij 76 phase diagram.23,35 However, the XRD pattern shows a high intensity reflection peak with the same lamellar unit cell parameter = 44 Å, Fig. 4(c). This suggests
that the mesoporous silica materials of the lyotropic liquid crystal solid mesophase have well-defined lamellar order. The broadening of the high intensity 100 reflection peak and the partial collapse in the low intensity d200 and d300 reflections may be ascribed to a lack of long-range crystallographic order or to finite size effects.6,20,36
°C has been established for lamellar phase materials.34 By increasing of the template concentration to 87%
(w/w) of Brij 76 (Table 1), the solid phase mesoporous material (S-SiO2) can be characterised in accord with the Brij 76 phase diagram.23,35 However, the XRD pattern shows a high intensity reflection peak with the same lamellar unit cell parameter = 44 Å, Fig. 4(c). This suggests
that the mesoporous silica materials of the lyotropic liquid crystal solid mesophase have well-defined lamellar order. The broadening of the high intensity 100 reflection peak and the partial collapse in the low intensity d200 and d300 reflections may be ascribed to a lack of long-range crystallographic order or to finite size effects.6,20,36 | ||
Fig. 4 XRD patterns of calcined mesoporous silicate materials synthesised at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C: (a) HI-SiO2, (b) L∞-SiO2 and (c) S-SiO2. °C: (a) HI-SiO2, (b) L∞-SiO2 and (c) S-SiO2. | ||
 | ||
Fig. 5 XRD patterns of calcined mesoporous silicate materials synthesised at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C: (a)
Ia3d-SiO2, (b)
Im3m-SiO2 and (c)
P63/mmc-SiO2.
°C: (a)
Ia3d-SiO2, (b)
Im3m-SiO2 and (c)
P63/mmc-SiO2. | ||
XRD patterns of the high temperature as-synthesised materials are observed to display several distinguishable Bragg peaks which can be related to different hkl reflections, Fig. 5. The mesoporous silica material produced by using 70% of Brij 76 at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C shows XRD reflection peaks that can be assigned to cubic lattices of space group symmetries Ia3d
(√6∶√8∶√14∶√16∶√20∶√22∶√24), Fig. 5(a).1,2,37 The presence of the finely resolved peaks with d-spacings of 46, 39.8, 30.0, 28.2, 25.2, 24.0 and 22.9 Å are similarly observed in MCM-48 mesoporous materials.38
°C shows XRD reflection peaks that can be assigned to cubic lattices of space group symmetries Ia3d
(√6∶√8∶√14∶√16∶√20∶√22∶√24), Fig. 5(a).1,2,37 The presence of the finely resolved peaks with d-spacings of 46, 39.8, 30.0, 28.2, 25.2, 24.0 and 22.9 Å are similarly observed in MCM-48 mesoporous materials.38
The XRD pattern (Fig. 5(b)) for the calcined mesoporous material prepared at high concentration of Brij 76 (76%), exhibits well-ordered reflection peaks of the cubic Im3m space group.27,39 These peaks display d-value ratios of √2∶√4∶√6∶√8∶√10∶√12∶√14, which are indicative of (110, 200, 211, 220, 310, 222, 321) reflections, respectively. This type of material is consistent with SBA-16 mesoporous silica material.39Fig. 5(c) shows the XRD patterns of the calcined mesoporous silica prepared in the presence of Brij 76 (81%) as template. Three poorly resolved peaks appear in the 2θ range 1–2° with d-spacings of 45.2, 43.5 and 39 Å. In addition, two resolved peaks in the 2θ range 3–5° with d-spacings of 25.2 and 21.3 Å are observed. These peaks correspond to the 100, 002, 101, 110 and 112 reflections, respectively. Such an XRD pattern is assigned to the three-dimensional p63/mmc hexagonal structure with unit cell parameters of, ψ = 55.2, c = 87 Å, with c/ψ = 1.58 which are consistent with the synthesised three-dimensional hexagonal mesophase, SBA-2.39,40 These results established that the three-dimensional hexagonal structure is formed from hexagonal close packing globular aggregate structures.41 Besides, the appearance of the low intensity 103 and 211 reflection peaks resembles that of well-defined long-range ordered mesoporous silica materials (p63/mmc-SiO2).
In the view of the XRD patterns, it may be concluded that the use of high synthesis temperatures leads to the formation of three-dimensional, bicontinuous and cylindrical mesoporous silicate structures rather than one-dimensional mesoporous materials.42
Transmission electron micrographs (TEM)
TEM was used to investigate the structure of the mesophase materials, as shown in Fig. 6. The calcined samples were utilised for the TEM studies since the stability of these samples enhanced the TEM images. | ||
| Fig. 6 TEM images of calcined mesoporous silicate materials: (a) HI-SiO2, (b) Im3m-SiO2, (c) Ia3d-SiO2, (d) L∞-SiO2, (e) S-SiO2 and (f) P63/mmc-SiO2. | ||
The TEM image for selected particles along the [100] direction in Fig. 6(a) shows regular arrays of uniform channels in a hexagonal texture. The separation distance between the channels is about 44 Å, which is consistent with the d100 X-ray reflection peak.2,43 The TEM image shown in Fig. 6(b), shows the mesoporous silica material (Im3m-SiO2). The characteristics of the TEM pattern suggests the presence of highly ordered three-dimensional cubic Im3m mesostructure.2,39 The selected view of the [100] projection in Fig. 6(c) shows a uniform pore structure along the [100] direction. These results are consistent with the bicontinuous cubic Ia3d liquid crystal phase that has been reported previously.1,43,44 Whilst it is difficult from one direction only of TEM images to definatively assign the three dimensional cubic Ia3d mesostructure,45 in this case the TEM images show several distinguishable X-ray reflection peeks of the cubic silica material (Ia3d-SiO2).46Fig. 6(d) and (e) shows the TEM patterns of the lamellar (L∞) and solid phase (S) mesoporous silica materials, respectively. Both images exhibit highly ordered layers indicating the existence of a lamellar lyotropic liquid crystal phase.47 The interlayer separation of both samples is about 40 Å, which is in good agreement with the position of the d100 reflection peak from X-ray analyses, Fig. 4(b) and (c).48
P63/mmc space symmetry and a three-dimensional hexagonal mesoporous silica structure have been confirmed for the TEM image of Fig. 6(f). However, the TEM patterns show a regular array of mesopores characteristic of the [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 23] orientation, which is indicative of 3-d hexagonal mesophases.39,49 In this case, well-ordered large channels are observed to be arranged in the same manner as 3-d hexagonal mesoporous silica materials of group symmetry (P63/mmc) that have been prepared in bulk silica mesophases.50–52
23] orientation, which is indicative of 3-d hexagonal mesophases.39,49 In this case, well-ordered large channels are observed to be arranged in the same manner as 3-d hexagonal mesoporous silica materials of group symmetry (P63/mmc) that have been prepared in bulk silica mesophases.50–52
Conclusion
The formation of well-defined long-range ordered mesoporous silica materials has been achieved by using high concentrations of non-ionic surfactant (Brij 76) and under strongly acidic conditions. The pre-existence of the lyotropic liquid crystal mesophases directed the formation of monolithic nanostructured materials through the liquid crystal template mechanism. The monolithic family includes materials with hexagonal (HI-SiO2), lamellar (L∞-SiO2), solid phase (S-SiO2), cubic (Ia3d-SiO2), cubic (Im3m-SiO2) and 3-d hexagonal (p63/mmc-SiO2) structure mesophases. At high temperature (ca. 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C), the formation of three-dimensional mesoporous structures is favoured.
°C), the formation of three-dimensional mesoporous structures is favoured.N2 adsorption/desorption isotherm curves established that all the synthesised materials have uniform mesophases without any micropore architictures. These materials have BET surface areas in the range 580–950 m2 g−1 and pore sizes of 34–39 Å. Such high surface areas and large pore sizes are a consequence of the relatively low synthesis temperatures. XRD and TEM analyses established reliable synthesis of these well-defined mesoporous silicates.
Acknowledgements
We thank Dr B. Cressey and C. P. Ship for their help with TEM measurements. We also appreciate Dr G. S. Attard for fruitful discussions.References
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olsan, E. W. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- Q. Huo, D. I. Morgalese, U. Ciesla, P. Y. Feng, T. E. Gier, P. Sieger, R. Leon, A. Firouzi, B. F. Chmelka, F. Schuth and G. D. Stucky, Nature, 1994, 368, 317 CrossRef CAS.
- Q. Huo, D. I. Morgalese, U. Ciesla, D. G. Demuth, P. Y. Feng, T. E. Gier, P. Sieger, R. Leon, P. M. Petroff, F. Schuth and G. D. Stucky, Chem. Mater., 1994, 6, 1176 CrossRef CAS.
- S. Inagaki, Y. Fukushima and K. Kuroda, J. Chem. Soc., Chem. Commun., 1993, 680 RSC.
- S. A. Bagshaw, E. Prouzet and T. J. Pinnavaia, Science, 1995, 269, 1242 CrossRef.
- P. T. Tanev and T. J. Pinnavaia, Science, 1995, 267, 865 CrossRef CAS.
- D. Y. Zhao and D. J. Goldfarb, J. Chem. Soc., Chem. Commun., 1995, 875 RSC.
- V. Alfredsson and M. W. Anderson, Chem. Mater., 1996, 8, 1141 CrossRef CAS.
- A. A. Romero, M. D. Alba and J. Klinowski, J. Phys. Chem. B, 1998, 102, 123 CrossRef CAS.
- A. A. Romero, M. D. Alba, W. Z. Zhou and J. Klinowski, J. Phys. Chem. B, 1997, 101, 5294 CrossRef CAS.
- M. Kruk, M. Jaroniec, R. Ryoo and J. M. Kim, Chem. Mater., 1999, 11, 2568 CrossRef CAS.
- A. Ayyappan and C. N. R. Rao, Chem. Commun., 1997, 575 RSC.
- A. Steel, S. W. Carr and M. W. Anderson, J. Chem. Soc., Chem. Commun., 1994, 1571 RSC.
- J. Luo and S. L. Suib, Chem. Commun., 1997, 1031 RSC.
- M. Ogawa, J. Am. Chem. Soc., 1994, 116, 7941 CrossRef CAS.
- G. S. Attard, J. C. Glyde and C. G. Göltner, Nature, 1995, 378, 366 CrossRef CAS.
- G. S. Attard, P. N. Bartlett, N. R. B. Coleman, J. M. Elliott, J. R. Owen and J. H. Wang, Science, 1997, 278, 838 CrossRef CAS.
- G. S. Attard, M. Edgar and C. G. Göltner, Acta Mater., 1998, 46, 751 CrossRef CAS.
- J. Evans, A. B. Zaki, M. Y. El-Sheikh and S. A. El-Safty, J. Phys. Chem. B, 2000, 104, 10271 CrossRef CAS.
- S. A. El-Safty, A. B. Zaki, M. Y. El-Sheikh and J. Evans, Colloids Surf., 2001, in press Search PubMed.
- X. S. Zhao, G. Q. Lu and G. J. Millar, Ind. Eng. Chem. Res., 1996, 35, 2075 CrossRef CAS.
- G. S. Attard, P. N. Bartlett, N. R. B. Coleman, J. M. Elliott and J. R. Owen, Langmuir, 1998, 14, 7340 CrossRef CAS.
- R. Schmidt, E. W. Hansen, M. Stocker, D. Akporiaye and O. H. Ellestad, J. Am. Chem. Soc., 1995, 117, 4049 CrossRef CAS.
- P. J. Branton, P. G. Hall, K. S. W. Sing, H. Reichert, F. Schuth and K. K. Unger, J. Chem. Soc., Faraday Trans., 1994, 90, 2965 RSC.
- S. J. Gregg and K. S. Sing, Adsorption, Surface Area and Porosity, 2nd edn., Academic Press, London, 1992 Search PubMed.
- P. Yang, D. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Nature, 1998, 152 CAS.
- P. L. Llewellyn, Y. Grillet, F. Schuth, H. Reichert and K. K. Unger, Microporous Mater., 1994, 3, 345 CrossRef CAS.
- S. S. Kim, W. Zhang and T. J. Pinnavaia, Science, 1998, 282, 1302 CrossRef CAS.
- D. Wei, H. Wang, X. Feng, W. Chuch, P. Ravikovitch, M. Lyubovsky, C. Li, T. Takeguchi and G. L. Haller, J. Phys. Chem. B, 1999, 103, 2113 CrossRef CAS.
- A. S. Bagshaw, E. Pruzet and T. J. Pinnavaia, Science, 1995, 269, 1242 CrossRef.
- S. Inagaki, A. Koiwai, N. Suzuki, Y. Fukushima and K. Kuroda, Bull. Chem. Soc. Jpn., 1996, 69, 1449 CAS.
- A. Firouzi, F. Atef, A. G. Oertli, G. D. Stucky and B. F. Chmekia, J. Am. Chem. Soc., 1997, 119, 3596 CrossRef CAS.
- Z. Luan, H. He, W. Zhou and J. Klinowski, J. Chem. Soc., Faraday Trans., 1998, 94, 979 RSC.
- D. J. Mitchell, J. T. Tiddy, L. Waring, T. Bostock and M. P. McDonald, J. Chem. Soc., Faraday Trans., 1983, 79, 975 RSC.
- C. Y. Chem, X. H. Li and M. E. Davis, Microporous Mater., 1993, 2, 27 CrossRef CAS.
- J. M. Kim, S. S. Kim and R. Ryoo, Chem. Commun., 1998, 259 RSC.
- J. Medina, J. A. Montoya and J. A. Reyes, Stud. Surf. Sci. Catal., 1998, 118, 889 Search PubMed.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- Q. Huo, D. I. Margolese and G. D. Stucky, Chem. Mater., 1996, 8, 1147 CrossRef CAS.
- H. Hagslatt, O. Soderman and B. Jonsson, Langmuir, 1994, 10, 2177 CrossRef.
- M. J. Kim and R. Ryoo, Chem. Mater., 1999, 11, 487 CrossRef.
- D. Zhao and D. Goldfarb, Stud. Surf. Sci. Catal., 1995, 97, 181 Search PubMed.
- J. Xu, Z. Luan, H. He, W. Zhou and L. Kevan, Chem. Mater., 1998, 10, 3690 CrossRef CAS.
- V. Alfredsson, M. W. Anderson, T. Ohsuna, O. Terasaki, M. Jacob and M. Bojrup, Chem. Mater., 1997, 9, 2066 CrossRef CAS.
- F. Chen, L. Huang and Q. Li, Chem. Mater., 1997, 9, 2685 CrossRef CAS.
- M. Templin, A. Franck, A. D. Chesne, H. Leist, Y. Zhang, R. Ulrich, V. Schädler and U. Wiesner, Science, 1997, 278, 1795 CrossRef CAS.
- S. Ayyappan and C. N. R. Rao, Chem. Commun., 1997, 575 RSC.
- Y. Lu, R. Ganguli, C. A. Drewien, M. T. Anderson, C. J. Brinker, W. Gong, Y. Guo, H. Soyes, B. Dunn, M. H. Huang and J. I. Zink, Nature, 1997, 389, 364 CrossRef CAS.
- Q. Huo, R.. Leon, P. M. Petroff and G. D. Stucky, Science, 1995, 268, 1324 CrossRef CAS.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525 RSC.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, Biochim. Biophys. Acta, 1977, 470, 185 CAS.
| This journal is © The Royal Society of Chemistry 2002 |
