Isotope ratio measurement by hexapole ICP-MS: mass bias effect, precision and accuracy
Qianli
Xie
* and
Robert
Kerrich
Department of Geological Sciences, University of Saskatchewan, Saskatoon, SK, Canada S7N 5E2. E-mail: xieq@duke.usask.ca; Tel: (306) 966-5691; Fax: (306) 966-8593
First published on 4th December 2001
Abstract
Isotope ratios were measured from the low mass (25Mg/26Mg) to the high mass (203Tl/205Tl) region, using an ELAN 5000 and a Platform Hex-ICP-MS, to investigate mass bias effects on the two instruments. For the ELAN 5000, the percentage mass discrimination per atomic mass unit (MD%) varies from 5% for 25Mg/26Mg to 0.7% for 203Tl/205Tl, whereas for the Platform it ranges from 10% to 0.11%, respectively. The Platform has higher mass bias at low mass and lower mass bias at the high mass region compared to the ELAN 5000. The difference in mass bias between the two instruments is attributed to the different ion transferring lens system, i.e., electrostatic lens system in the ELAN 5000 and hexapole collision cell in the Platform. For the Platform Hex-ICP-MS, the addition and variation of collision/reaction gases (He and H2) does not introduce any additional mass bias. The precision and accuracy of isotope ratio measurement by the Platform Hex-ICP-MS were assessed using the NBS982 Pb isotope reference material and an elemental Sn standard spiked with 117Sn-enriched spike. The overall precision was found to be 0.1–0.6% relative standard deviation.
Introduction
Precise and accurate isotope ratio measurements have traditionally been carried out by thermal ionization mass spectrometry (TIMS). In the past few years inductively coupled plasma source mass spectrometry (ICP-MS), with both a quadrupole mass analyzer-single collector (Q-ICP-MS) and a magnetic sector mass analyzer, single or multiple collector configuration (MC-ICP-MS), have increasingly been used in high precision and accuracy isotope ratio measurement.1–3 Although Q-ICP-MS cannot achieve the precision and accuracy in isotope ratio measurement attainable by TIMS and MC-ICP-MS, the technique can still play an important role in isotope ratio measurement for such applications as isotope dilution analysis, isotope labelled metal bioavailability and toxicity studies, metal speciation studies and natural isotope variations.2,4–6Several aspects of instrument performance negatively affect the precision and accuracy of isotope ratio measurement by ICP-MS: specifically poor sensitivity, signal stability, spectral interferences, and mass discrimination. One way to reduce or eliminate spectral interference is to employ a high resolution magnetic sector mass analyzer. However, a drawback of the high resolution mass analyzer is the inherent decrease in instrument sensitivity.2 Alternatively, a “chemical resolution” technique has been successfully applied to quadrupole ICP-MS. The technique utilizes a “collision” or “reaction” cell filled with various gases (e.g., He, H2, NH3 or Xe) to reduce or eliminate spectral interferences.7–12 In most current models of ICP-MS that utilize the “chemical resolution” technique, the collision/reaction cell also acts as ion focusing optics that homogenize ion energy and hence increase ion transmission efficiency.9,10,13
Mass bias is the deviation of measured isotope ratios by a mass spectrometer from the “true value”, due to mass discrimination that occurs in all mass spectrometers. In particular, several factors have been found to affect bias in ICP-MS, such as detector “dead time”, the electrostatic ion focusing lens and the “space charge” effect occurring at the interface region.1,14 In general, mass bias in TIMS is time dependent, whereas mass bias change with time in ICP-MS has been demonstrated to be much slower under given instrumental parameters such as nebulization gas flow rate, torch position, and rf power. Hence, mass bias in ICP-MS can be predicted and more accurate corrections can be applied.15 However, with the addition of a “collision cell”, some concerns have arisen as to whether this introduces unpredictable mass bias effecs.16,17
In this study, isotope ratios were measured at low and high mass regions, using a conventional ICP-MS and a hexapole “collision cell” ICP-MS, to compare the mass bias of the two. The effect of varying collision gases on the mass bias of the hexapole ICP-MS (Hex-ICP-MS) was also investigated. The precision and accuracy of isotope ratio measurement using a Hex-ICP-MS was assessed by the measurement of Pb isotope ratios in NBS-982 standard, and the Sn isotope composition of an elemental Sn standard with natural isotope abundance, but spiked with 117Sn-enriched isotope in various proportions.
Experimental
Samples and reagents
Pure elemental standards (Inorganic Ventures, Inc., Lakewood, NJ, USA) were used to prepare the diluted multi-element solutions. For Sn isotope ratios, in addition to diluted pure elemental standard, three solutions of pure elemental standard spiked with 117Sn-enriched spike (Oak Ridge Laboratory, USA) in different ratios were also prepared. Pb isotope ratios were measured using NBS 982 standard solution (NIST, Gaithersburg, MD, USA). Double distilled HNO3 and deionized water (>18.2 MΩ, Barnstead, Dubuque, IA, USA) were used for sample dilution. All sample preparations were carried out in a clean room environment.Instrumentation
The ICP-MS instruments used in this study were a PerkinElmer Elan 5000 (PE-Sciex, Concord, ON, Canada) and a Micromass Platform (Manchester, UK). The former is a conventional ICP-MS with four ion focusing lenses and a photon stop, whereas the latter is equipped with a hexapole collision/reaction cell. The collision cell filled with gases at 10−3 mbar (e.g., He and H2) serves two functions: (1) reduction and elimination of Ar-based polyatomic interferences through collisional dissociation and reaction; and (2) that of an ion focusing lens through thermalization of ions that reduce the dispersion of ion energy, thus enhancing ion transmission.9,10,12–14 Schematics of instrumental configuration are shown in Fig. 1. A standard sample introduction system consisting of a Meinhard-type nebulizer, a Scott type double path spray chamber, and a Fassel quartz torch were used on both instruments. Operation parameters for both instruments are listed in Table 1. | ||
| Fig. 1 Schematics of instrument configuration of the Elan 5000 (a) and Platform ICP-MS (b). | ||
| a Instrument read-back. | |
|---|---|
| PE Elan 5000— | |
| Rf forward power | 1100 W |
| Cool gas flow rate | 10 L min−1 |
| Intermediate gas flow rate | 0.8 L min−1 |
| Nebulizer gas flow rate | 0.76 L min−1 |
| Sample uptake rate | 1 ml min−1 |
| Sampler/skimmer cones | Ni, 1.1 and 0.89 mm diameter, respectively |
| Lens settings/Va | P: −69, B: +9.1, S2: −8.3, E1: +8.2 |
| Acquisition | Peak hopping |
| Dwell time | 30 ms peak |
| Point peak | 1 |
| Micromass platform— | |
| Rf forward power | 1350 W |
| Cool gas flow rate | 14 L min−1 |
| Intermediate gas flow rate | 0.85 L min−1 |
| Nebulizer gas flow rate | 0.76 L min−1 |
| Sample uptake rate | 0.70 ml min−1 |
| Sampler/skimmer cones | Ni, 1.1 and 0.7 mm diameter, respectively |
| Extraction lens | −400 V |
| Exit lens | 400 V |
| Hexapole bias | −1.6 V |
| Ion energy | 2 V |
| Multiplier | 450 V |
| He gas flow rate | Variable, optimum 5.0 ml min−1 |
| H2 gas flow rate | Variable, optimum 2.8 ml min−1 |
| Acquisition mode | Single ion measuring (peak hopping) |
| Dwell time | 200 ms |
| Point/peak | 1 |
Results
Table 2 presents the results of isotope ratio measurements from low mass (Mg) to high mass (Tl) by the Elan 5000 and Platform. Also listed in the table are mass bias factors (fMD) and percentage mass discrimination per atomic mass unit (MD%). The fMD and MD% are defined as follows:| fMD = Rtrue/Rmeasured | (1) |
| MD(%) = (fMD−1) × 100/Δm | (2) |
| Platformb | 25Mg/26Mg | 63Cu/65Cu | 86Sr/88Sr | 95Mo/96Mo | 107Ag/109Ag | 151Eu/153Eu | 184W/186W | 203Tl/205Tl |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.8218 | 2.0278 | 0.1147 | 0.9427 | 1.0476 | 0.9056 | 1.0790 | 0.4186 |
| 2 | 0.8210 | 2.0054 | 0.1148 | 0.9438 | 1.0424 | 0.9118 | 1.0689 | 0.4148 |
| 3 | 0.8234 | 1.9939 | 0.1143 | 0.9422 | 1.0385 | 0.9060 | 1.0721 | 0.4170 |
| 4 | 0.8318 | 2.0053 | 0.1162 | 0.9480 | 1.0450 | 0.9065 | 1.0671 | 0.4182 |
| 5 | 0.8228 | 2.0107 | 0.1149 | 0.9344 | 1.0472 | 0.9042 | 1.0776 | 0.4228 |
| 6 | 0.8132 | 2.0178 | 0.1142 | 0.9358 | 1.0359 | 0.9052 | 1.0734 | 0.4139 |
| 7 | 0.8213 | 2.0285 | 0.1154 | 0.9267 | 1.0384 | 0.9032 | 1.0816 | 0.4202 |
| 8 | 0.8209 | 2.0147 | 0.1144 | 0.9386 | 1.0452 | 0.9069 | 1.0734 | 0.4169 |
| 9 | 0.8168 | 2.0243 | 0.1140 | 0.9323 | 1.0457 | 0.9001 | 1.0682 | 0.4192 |
| 10 | 0.8232 | 2.0111 | 0.1145 | 0.9433 | 1.0488 | 0.9096 | 1.0696 | 0.4184 |
| Average | 0.8216 | 2.0139 | 0.1147 | 0.9388 | 1.0435 | 0.9059 | 1.0731 | 0.4180 |
| Standard | 0.0048 | 0.0110 | 0.0007 | 0.0065 | 0.0044 | 0.0032 | 0.0049 | 0.0026 |
| RSD (%) | 0.58 | 0.55 | 0.58 | 0.69 | 0.43 | 0.36 | 0.46 | 0.61 |
| f MD | 1.1055 | 1.1140 | 1.0406 | 1.0167 | 1.0315 | 1.0112 | 1.0043 | 1.0022 |
| MD(%) | 10.58 | 5.71 | 2.03 | 1.67 | 1.58 | 0.56 | 0.22 | 0.11 |
| Elan 5000 | 25Mg/26Mg | 63Cu/65Cu | 86Sr/88Sr | 95Mo/96Mo | 107Ag/109Ag | 151Eu/153Eu | 184W/186W | 203Tl/205Tl |
|---|---|---|---|---|---|---|---|---|
| a 10 ppb solution for Platform and 100 ppb for Elan 5000. b Collision gas settings: He = 5.0 ml min−1, H2 = 2.8 ml min−1. c From IUPAC (1997). | ||||||||
| 1 | 0.8669 | 2.0332 | 0.1139 | 0.9466 | 1.0139 | 0.8772 | 1.0640 | 0.4265 |
| 2 | 0.8412 | 2.0388 | 0.1211 | 0.9179 | 1.0449 | 0.8896 | 1.0256 | 0.3972 |
| 3 | 0.9357 | 1.9527 | 0.1155 | 0.9049 | 1.0361 | 0.8720 | 1.0696 | 0.4042 |
| 4 | 0.9342 | 2.1382 | 0.1137 | 0.9556 | 1.0524 | 0.8567 | 1.0466 | 0.3992 |
| 5 | 0.8406 | 1.9774 | 0.1161 | 0.9431 | 1.0174 | 0.8959 | 1.0640 | 0.4224 |
| 6 | 0.8519 | 2.0064 | 0.1127 | 0.9366 | 1.0497 | 0.8797 | 1.0576 | 0.4103 |
| 7 | 0.8621 | 2.0363 | 0.1100 | 0.9210 | 0.9914 | 0.8733 | 1.0986 | 0.4105 |
| 8 | 0.8349 | 2.1430 | 0.1131 | 0.9266 | 1.0418 | 0.8875 | 1.0777 | 0.4048 |
| 9 | 0.8462 | 1.9467 | 0.1041 | 0.8725 | 1.0149 | 0.8961 | 1.0609 | 0.4226 |
| 10 | 0.8216 | 2.0532 | 0.1139 | 0.9430 | 1.0362 | 0.8789 | 1.0538 | 0.4303 |
| Average | 0.8635 | 2.0326 | 0.1134 | 0.9268 | 1.0299 | 0.8807 | 1.0618 | 0.4128 |
| Standard | 0.0398 | 0.0677 | 0.0043 | 0.0245 | 0.0196 | 0.0121 | 0.0192 | 0.0118 |
| RSD | 4.61 | 3.33 | 3.83 | 2.64 | 1.90 | 1.38 | 1.80 | 2.87 |
| f MD | 1.0518 | 1.1038 | 1.0528 | 1.0298 | 1.0452 | 1.0402 | 1.0150 | 1.0148 |
| MD(%) | 5.19 | 5.20 | 2.65 | 2.99 | 2.26 | 2.01 | 0.75 | 0.74 |
| Natural ratioc | 0.9083 | 2.2436 | 0.1194 | 0.9544 | 1.0764 | 0.9161 | 1.0777 | 0.4189 |
For the Platform ICP-MS, the relative standard deviation (RSD) of measured isotope ratios varies between 0.4 and 0.7%. In all isotope ratios fMD is >1, consistent with light isotopes being transmitted less efficiently in ICP-MS.19 The mass discrimination per atomic mass unit (MD%) varies from 10% for 25Mg/26Mg to 0.11% for 203Tl/205Tl, similar to previously determined MD% values by the same type of instrument.2
For the Elan 5000 the RSD of isotope ratio measurement is between 2 and 5%, significantly higher than that of the Platform, despite the elemental concentration in the analyte being higher for the Elan 5000 than for the Platform. The mass bias factor (fMD) is also >1, and the MD% varies from 5% for 25Mg/26Mg to 0.7% for 203Tl/205Tl, similar to those reported by Heumann et al.1 on an Elan 5000.
There is also a difference in MD(%) between the Elan 5000 and Platform (Table 2 and Fig. 2). Specifically, the Platform appears to have larger MD% at low mass, but smaller MD% at high mass compared with the Elan 5000. Heumann et al.1 also found differences in mass bias among three quadrupole ICP-MS instruments (Spectromass 2000, Elan 5000, and HP4500), and attributed the difference to the different instrument design.1
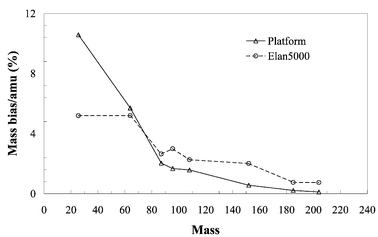 | ||
| Fig. 2 Comparison of mass bias per amu versus mass between the Elan 5000 and Platform ICP-MS. See text for details of measurement and instrument parameters. | ||
Discussion
Detector dead time
Elan 5000 uses a channel electron multiplier and a pulse counting system for ion signal measurement. At a high count rate, a continuous dynode electron multiplier may register fewer counts than actually occur, a phenomenon termed “detector dead time”.19 A consequence of detector dead time in isotope ratio measurement is the deviation of measured ratio from “true ratio” due to the dead time effect in measuring the more abundant isotope, and a correction may be necessary. For the Elan 5000 used in this study, Xie and Kerrich20 found that measured 91Zr/90Zr ratios were within error at analyte concentrations between 80 and 1000 ng g−1 (equivalent to 105–106 counts s−1), suggesting that at this count rate dead time correction is not necessary. The analyte concentration used for the Elan 5000 in this study was 100 ng ml−1 (equivalent to 1 × 105 counts s−1).The Platform uses a “Daly-type photomultiplier” with a linearity up to 1 × 108 counts s−1. At count rates >1 × 108, a strong “after glow” will occur, and subsequent counts will not be registered, a phenomenon similar to the dead time in a continuous dynode electron multiplier. Fig. 3 shows the instrument response with varying Ba concentration measured on the Platform ICP-MS. The data indicates that the instrument response remains linear up to 2 × 106 counts s−1, corresponding to a Ba concentration of 10 ng g−1. The analyte signal on the Platform in this study was between 1 × 105 and 1 × 106. Thus, a detector “dead time” correction was considered unnecessary.10 Accordingly, detector dead time is unlikely to be the reason that the two instruments have different mass bias.
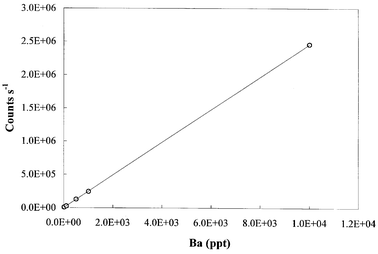 | ||
| Fig. 3 Count rate versus Ba concentration measured by the Platform, showing linear response of the detector up to 2 × 106 counts s−1. | ||
Mass bias in ICP-MS with and without collision cell
Two major factors have been found to affect mass discrimination in ICP-MS: (1) electrostatic ion focusing lens; and (2) “space charge” and “nozzle separation” effect at the interface region.1,14 Mass discrimination occurs when ion transmission efficiency in the ion transfer lens system is different with different ion energies. A plasma source produces ions with a large energy spread. In a particular ion transfer lens system, there will usually be an optimum lens setting that will transfer ions with a certain energy more efficiently than ions with different energy, introducing mass discrimination against either light ions or heavy ions, depending on the optimum focal energy.14,21 For example, Xie and Kerrich20 found that changing the B lens setting of the Elan 5000 from +6 to +14 V (40–80 digipots), 90Zr/91Zr and 178Hf/179Hf ratios first increase, and then decrease, after an optimum setting of 60. They attributed such change in mass discrimination to the different energy focal point under different lens settings.20 Other studies have also shown the effects of ion lens settings on mass bias in ICP-MS.1,22 Such mass discrimination induced by the ion transfer lens system may be against light or heavy isotopes depending on ion energy. This mass discrimination effect is different from the mass discrimination caused by “space charge” and “nozzle separation” effects that occurs in the interface region.In all ICP-MS designs, there is a substantial column of positively charged particles behind sampler and skimmer cones; positively charged particles are mutually repelled. In this region, the light ions tend to disperse more than heavy ions, resulting in mass discrimination against light isotopes. This is termed “space charge” effect.23 More recently, Heumann et al.1 suggested that when a supersonic jet of charged particles passes through the ICP-MS interface region, light isotopes should preferably be pumped down and therefore more light isotopes leave the central beam compared with the heavier isotopes. They termed this the “nozzle separation” effect. “Nozzle separation” effect also introduces mass discrimination against light ions. However, because the “space charge” and “nozzle separation” produce mass bias in the same direction, it is difficult to distinguish the two. The “space charge” effect has been considered to have the strongest mass bias effect in ICP-MS, inducing a mass discrimination consistently against the light isotope.23
For an ICP-MS with an ion focusing lens system, such as the Elan 5000, the total bias is a combination of ion lens system and “space charge/nozzle separation” effects, and is dependent on ion mass and energy. In contrast, for the Platform ICP-MS equipped with a collision cell, the total bias is dominated by “space charge” and “nozzle separation” effect. Furthermore, the collision cell also homogenizes ion energy, reducing energy spread to <1 eV.24 Hence, the total bias in a Platform is dependent on mass, but independent of ion energy. This is, perhaps, the reason that the Elan 5000 has lower apparent mass bias at low mass, but higher apparent bias at high mass (Fig. 2), whereas the Platform appears to have mass bias that is correlated exponentially with mass (Fig. 2). Palacz recently also showed the different mass bias between a hexapole single focusing MC-ICP-MS and a double focusing MC-ICP-MS, and suggested that the ESA employed in a double focusing MC-ICP-MS may offset the bias–mass correlation.15 The exponential law has been considered a good approximation for mass bias occurring in an ICP-MS interface.25–29
Effect of varying collision gas on mass bias
As described above, the Platform uses a collision cell filled with gases as an ion transfer system, instead of an electrostatic ion focusing lens system, to guide ions to the quadrupole mass filter. The addition of He and H2 serves two functions: (1) thermalization of ions to reduce ion energy spread; (2) reduction of Ar-based polyatomic interference through collisional dissociation and reaction. A question arises as to whether additional mass bias is induced by hexapole gases. To investigate the effect of collision gases on instrumental mass bias, isotope ratios ranging from 25Mg/26Mg to 203Tl/205Tl were measured with variable gas settings.Table 3 and Fig. 4 present the fMD and MD(%) of various isotope ratios measured under different gas settings. All other parameters remain the same (see Table 1). The fMD varies from 1.1 at low mass (25Mg/26Mg) to 1.002 at high mass (203Tl/204Tl). There is no significant difference in bias per amu under different gas settings, except for 25Mg/26Mg, where MD% changes from 10.5 under He = 5.0 ml min−1 and H2 = 2.8 ml min−1 to 7.7 under He = 5.0 ml min−1 and no H2 (Table 3, Fig. 4). These results suggest that change of hexapole gas settings does not significantly affect mass bias in the Platform. In contrast, changing lens setting in a conventional ICP-MS with an electrostatic ion focus lens system can induce mass bias against light or heavy isotopes depending on ion energy.14,19,20,22,23 It appears that in hexapole ICP-MS, mass bias occurs predominantly in the interface region induced by “space charge” and “nozzle separation” effects.
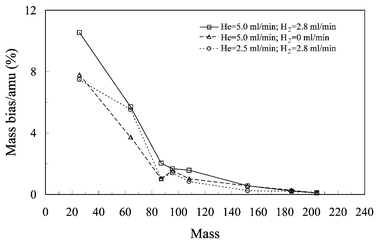 | ||
| Fig. 4 Mass bias per amu (MD%) under three different hexapole gas settings for the Platform. The three settings are: (□) He = 5.0 ml min−1 and H2 = 2.8 ml min−1; (△) He = 5.0 ml min−1 and H2 = 0 ml min−1; and (○) He = 2.5 ml min−1 and H2 = 2.8 ml min−1. Other parameters remain the same (see Table 1 and text for details). | ||
| 25Mg/26Mg | 63Cu/65Cu | 86Sr/88Sr | 95Mo/96Mo | 107Ag/109Ag | 151Eu/153Eu | 184W/186W | 203Tl/205Tl | |
|---|---|---|---|---|---|---|---|---|
| a Measured with 10 ppb multi-element solution. | ||||||||
| He = 5.0 ml min−1; H2 = 2.8 ml min−1 | ||||||||
| f MD | 1.1055 | 1.1140 | 1.0406 | 1.0167 | 1.0315 | 1.0112 | 1.0043 | 1.0022 |
| MD(%) | 10.55 | 5.70 | 2.03 | 1.67 | 1.58 | 0.56 | 0.22 | 0.11 |
| He = 5.0 ml min−1; H2 = 0 ml min−1 | ||||||||
| f MD | 1.0779 | 1.0748 | 1.0203 | 1.0156 | 1.0203 | 1.0111 | 1.0054 | 1.0017 |
| MD(%) | 7.79 | 3.74 | 1.02 | 1.56 | 1.01 | 0.55 | 0.27 | 0.09 |
| He = 5.0 ml min−1; H2 = 2.8 ml min−1 | ||||||||
| f MD | 1.0748 | 1.1104 | 1.0204 | 1.0140 | 1.0168 | 1.0047 | 1.0039 | 1.0020 |
| MD(%) | 7.48 | 5.52 | 1.02 | 1.40 | 0.84 | 0.24 | 0.19 | 0.10 |
Precision and accuracy
Two measurements were made to assess the precision and accuracy of isotope ratio determination attainable with a hexapole ICP-MS. The 117Sn/120Sn ratio was measured on three solutions (S1, S2 and S3). The three solutions were prepared from pure elemental Sn solution spiked with 117Sn-enriched isotope spike, with final Sn concentrations of about 6, 11 and 16 ppb, respectively. The spiked ratios based on gravimetric sample preparation are listed in Table 4. Also listed in Table 4 are the signal intensity and bias corrected measured 117Sn/120Sn ratios. A separate 10 ppb Sn solution with natural isotopic composition was also measured to determine mass bias. Natural Sn isotope composition from IUPAC18 was used to calculate mass bias factor of 117Sn/120Sn, and the calculated mass bias factor was used for bias correction of samples.| Measurement # | S1 (6 ppb) | S2 (11 ppb) | S3 (16 ppb) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 117 cpsb | 120 cps | 117Sn/120Snc | 117 cps | 120 cps | 117Sn/120Snc | 117 cpsb | 120 cps | 117Sn/120Snc | |
| a Measured with: dwell time 0.2 s; inter-channel delay 0.02 s; 1 acquisition/measurement, total time/measurement 1 min. Other parameters as in Table 1. b Counts per second. c Bias corrected ratios. See text for details. | |||||||||
| 1 | 287997 | 295552 | 1.0014 | 363128 | 602349 | 0.6195 | 411103 | 863655 | 0.4892 |
| 2 | 282433 | 291080 | 0.9971 | 352519 | 587534 | 0.6166 | 403128 | 849517 | 0.4877 |
| 3 | 277396 | 287252 | 0.9924 | 351191 | 584561 | 0.6174 | 405763 | 851065 | 0.4900 |
| 4 | 279189 | 286293 | 1.0022 | 350850 | 583329 | 0.6181 | 410988 | 861023 | 0.4889 |
| 5 | 273643 | 280157 | 1.0038 | 353887 | 588990 | 0.6175 | 407849 | 856734 | 0.4889 |
| Average | 280132 | 288067 | 0.9994 | 354315 | 589353 | 0.6178 | 407766 | 856399 | 0.4889 |
| Standard deviation | 5423 | 5733 | 0.0046 | 5071 | 7609 | 0.0011 | 3430 | 6123 | 0.0008 |
| Relative standard deviation (%) | 1.94 | 1.99 | 0.46 | 1.43 | 1.29 | 0.18 | 0.84 | 0.71 | 0.17 |
| Spiked ratio | 1.0103 | 0.6236 | 0.4931 | ||||||
| Deviation from true ratio | −0.0109 | −0.0058 | −0.0041 | ||||||
The precision of 117Sn/120Sn ratio based on 5 measurements ranges from 0.46% in S1 to 0.17% in S3. The accuracy, measured by the difference between the bias-corrected observed ratio and the spike-adjusted, true ratio, ranges from −0.0109 in S1 to −0.0041 in S3 (Table 4). The difference between bias corrected and spiked ratios suggests that there is still a residual error in bias correction. Such residual error most likely stems from the external mass bias correction, i.e., mass bias factors were measured on a different solution and applied to samples assuming they have similar mass bias. As mass bias in ICP-MS occurs predominantly in the interface region and is dependent on sample matrix, such external correction may not fully correct the bias effect. The experiments indicated that the instrument mass bias was stable over 4 h.
Internal correction is also commonly used in isotope ratio measurement by mass spectrometry. For example, in Nd isotope ratio measurement, mass bias is determined using 146Nd/144Nd and applied to other isotope ratios (e.g., 143Nd/144Nd) in the same sample. In cases where there is no pair of stable isotope (e.g., Pb) or all isotopes are to be measured (Ca, Fe, Cu, etc.), a different element close in mass can be used. For example, Tl has been used for Pb isotopes,22 and Zn for Cu isotopes.29Table 5 presents Pb isotope measurement of the standard NBS982 by hexapole ICP-MS, using Tl for internal mass bias correction. An NBS982 solution with 20 ppb Pb was spiked with 10 ppb high purity Tl solution (Inorganic Ventures, Inc.). Mass bias factor was determined using measured 203Tl/205Tl and the IUPAC value (0.4189), and applied to Pb isotope ratios using an exponential correction law.25
| Measurement # | 206Pb/204Pb | 207Pb/204Pb | 208Pb/204Pb | 207Pb/206Pb | 208Pb/206Pb | 208Pb/207Pb |
|---|---|---|---|---|---|---|
| a Ratios were corrected for mass bias using the 203Tl/205Tl ratio. See text for details. | ||||||
| 1 | 36.8380 | 17.0503 | 35.9616 | 0.4628 | 0.9910 | 2.1411 |
| 2 | 36.3725 | 16.8636 | 35.6922 | 0.4636 | 0.9953 | 2.1468 |
| 3 | 36.4566 | 17.0327 | 35.9056 | 0.4672 | 1.0013 | 2.1432 |
| 4 | 36.5009 | 17.0850 | 35.7566 | 0.4681 | 0.9978 | 2.1317 |
| 5 | 36.6340 | 17.0631 | 35.9459 | 0.4658 | 0.9953 | 2.1369 |
| 6 | 36.7084 | 17.0923 | 36.1047 | 0.4656 | 0.9995 | 2.1465 |
| 7 | 36.5952 | 17.2067 | 36.1416 | 0.4702 | 1.0018 | 2.1306 |
| 8 | 36.9915 | 17.2687 | 36.4923 | 0.4668 | 1.0027 | 2.1479 |
| 9 | 36.6806 | 17.1249 | 36.2489 | 0.4669 | 1.0023 | 2.1470 |
| 10 | 36.4334 | 17.0139 | 35.7641 | 0.4670 | 0.9956 | 2.1319 |
| 11 | 35.8323 | 16.8769 | 36.0798 | 0.4710 | 1.0069 | 2.1378 |
| 12 | 36.2122 | 16.9513 | 36.1913 | 0.4681 | 0.9994 | 2.1350 |
| 13 | 36.1443 | 16.9717 | 35.8753 | 0.4696 | 0.9926 | 2.1138 |
| 14 | 36.1170 | 16.9091 | 36.1563 | 0.4682 | 1.0011 | 2.1383 |
| 15 | 36.2262 | 16.9605 | 36.5097 | 0.4682 | 1.0078 | 2.1526 |
| 16 | 35.9458 | 16.7921 | 36.1034 | 0.4672 | 1.0044 | 2.1500 |
| 17 | 36.3160 | 17.0106 | 36.4107 | 0.4684 | 1.0026 | 2.1405 |
| 18 | 36.3110 | 16.9584 | 36.4819 | 0.4670 | 1.0047 | 2.1513 |
| 19 | 35.9790 | 16.9331 | 36.5892 | 0.4706 | 1.0170 | 2.1608 |
| 20 | 36.2698 | 16.9670 | 36.1680 | 0.4678 | 0.9972 | 2.1317 |
| 21 | 36.0520 | 16.8923 | 36.0346 | 0.4686 | 0.9977 | 2.1332 |
| Average | 36.3627 | 17.0011 | 36.1245 | 0.4676 | 1.0007 | 2.1404 |
| Standard deviation | 0.3053 | 0.1147 | 0.2611 | 0.0020 | 0.0058 | 0.0102 |
| Relative standard deviation (%) | 0.84 | 0.67 | 0.72 | 0.43 | 0.58 | 0.48 |
| Certified values | 36.7385 | 17.1595 | 36.7443 | 0.4671 | 1.0002 | 2.1413 |
| Deviation from true ratios | −0.3757 | −0.1583 | −0.6199 | 0.0005 | 0.0005 | −0.0009 |
The precision of bias corrected Pb isotope ratios ranges from 0.43% to 0.84% (Table 5). The RSD% for ratios involving 204Pb is higher than other isotope ratios. This is due to: (1) lower abundance in 204Pb; and (2) background correction of Hg contribution on mass 204. The deviation from the certified value ranges from 0.0005 (0.1%) for 207Pb/206Pb to −0.6199 (1%) for 208Pb/204Pb. The accuracy is also poorer for ratios involving 204Pb. A precision of 0.1% RSD or less has been achieved on Pb isotope ratio measurement of NBS981 with 100 ng g−1 Pb on a Platform.10 Mason et al. obtained a precision of 0.03–0.05% in 34S/32S measurement of 10–50 mg L−1 solutions using a Platform with He, H2 and Xe as collision gases.11
Conclusions
This study suggests that mass bias in hexapole ICP-MS appears to occur predominantly in the interface region, and has a bias–mass relation in agreement with previously established exponential form (Fig. 2). Compared with conventional ICP-MS, the hexapole ICP-MS has high mass bias at low mass (Mg) region, but lower mass bias at higher mass. Addition of hexapole gases (e.g., He and H2) does not introduce a significant mass bias effect, in contrast to conventional ICP-MS equipped with an electrostatic ion focusing lens system, which may induce mass bias against light or heavy isotopes. The precision and accuracy were assessed by the measurement of 117Sn/120Sn ratios on natural Sn solutions spiked with 117Sn-enriched spike, and the measurement of Pb isotopes on NBS982 using Tl for internal mass bias correction. The measured isotope ratios agree well with the expected values. For example, the measured 207Pb/206Pb on NBS982 is 0.46755, compared with a certified value of 0.46707. The overall precision of isotope ratio measurement by hexapole ICP-MS in this study is 0.2–0.6%.Acknowledgements
The Platform Hex-ICP-MS was purchased with a Natural Science and Engineering Research Council of Canada (NSERC) equipment grant. This work was supported by an NSERC Major Facility Access grant, University of Saskatchewan, and the George McLeod endowment to the Department of Geological Sciences. We thank Fadi R. Abou-Shakra and Micromass Ltd. staffs for their help and continuous support. Incisive critiques and suggestions from the two journal reviewers have greatly improved the manuscript.References
- K. G. Heumann, S. M. Gallus, G. Radlinger and J. Vogl, J. Anal. At. Spectrom., 1998, 13, 1001 RSC.
- J. S. Becker and H. J. Dietze, Fresenius' J. Anal. Chem., 2000, 368, 23 CrossRef CAS.
- A. N. Halliday, D.-C. Lee, J. N. Christensen, M. Rehkamper, W. Yi, X. Luo, C. M. Hall, C. J. Ballentine, T. Pettke and C. Sterling, Geochim. Cosmochim. Acta, 1998, 62, 919 CrossRef CAS.
- H. P. Longerich, At. Spectrosc., 1989, 10, 112 Search PubMed.
- Q. Xie and R. Kerrich, Chem. Geol., 1995, 123, 17 CrossRef CAS.
- I. Horn, R. L. Rudnick and W. F. McDonough, Chem. Geol., 2000, 164, 281 CrossRef CAS.
- D. J. Douglas, Can. J. Spectrosc., 1989, 34, 38 Search PubMed.
- G. C. Eiden, D. J. Barinaga and D. W. Koppenaal, J. Anal. At. Spectrom., 1996, 11, 317 RSC.
- I. Feldmann, N. Jakubowski and D. Stuewer, Fresenius' J. Anal. Chem., 1999, 365, 415 CrossRef CAS.
- I. Feldmann, N. Jakubowski, C. Thomas and D. Stuewer, Fresenius J. Anal. Chem., 1999, 365, 422 CrossRef CAS.
- P. R. Mason, K. Kaspers and M. J. van Bergen, J. Anal. At. Spectrom., 1999, 14, 1067 RSC.
- S. D. Tanner, V. I. Baranov and U. Vollkopf, J. Anal. At. Spectrom., 2000, 15, 1261 RSC.
- D. R. Bandura, V. I. Baranov and S. D. Tanner, Fresenius' J. Anal. Chem., 2001, 370, 454 Search PubMed.
- P. J. Turner, in G. Holland and A. Eaton, Applications of Plasma Source Mass Spectrometry, Royal Society of Chemistry, Cambridge, 1991, 71–78 Search PubMed.
- Z. Palacz, Ultra-high Precision Neodymium Isotope Ratio Measurements Using the Micromass Isoprobe, the Single Focussing Inductively Coupled Plasma Source Mass Spectrometer (ICP-MC-MS). Poster, European Union of Geosciences 2001, Strasbourg, France, 8–12 April, 2001.
- D. Bandura, V. I. Baranov and S. D. Tanner, J. Anal. At. Spectrom., 2000, 15, 921 RSC.
- F. Gouteland, F. Chartier and D. Vailhen, Precise and Accurate Isotopic Measurement Using a Multi-collector ICP-MS. Abstract, Geoanalysis 2000, Nancy, France, August 29–September 1, 2000.
- K. J. R. Rosman and P. D. P. Taylor, J. Phys. Chem. Ref. Data, 1998, 27, 1275 CAS.
- K. E. Jarvis, A. L. Gray and R. S. Houk,Handbook of Inductively Coupled Plasma Mass Spectrometry, Blackie, Glasgow and London, 1992, p. 376 Search PubMed.
- Q. Xie and R. Kerrich, J. Anal. At. Spectrom., 1995, 10, 99 RSC.
- P. H. Dawson, Quadrupole Mass Spectrometry and its Applications, Elsevier, Amsterdam, 1976, p. 143 Search PubMed.
- H. P. Longerich, B. J. Fryer and D. F. Strong, Spectrochim. Acta, 1987, 42B, 39 Search PubMed.
- G. P. Russ III and J. M. Bazan, Spectrochim. Acta, 1987, 42B, 49 Search PubMed.
- P. J. Turner, T. O. Merren, J. Speakman, R. C. Haines, Z. Palacz and S. Meffan-Main, Ion Optics of Multi-collector ICP-MS systems for Precise and Accurate Isotope Ratio Measurement, Technical Note 403, Micromass Ltd., Manchester, UK, 2000, pp. 1–11 Search PubMed.
- W. A. Russell, D. A. Papanastassiou and T. A. Tombrello, Geochim. Cosmochim. Acta, 1978, 42, 1075 CrossRef CAS.
- W. M. White, F. Albarede and P. Telouk, Chem. Geol, 2000, 167, 257 CrossRef CAS.
- J. Blichert-Toft, C. Chauvel and F. Albarede, Contrib. Mineral. Petrol., 1997, 127, 248 CrossRef CAS.
- B. Luais, P. Telouk and F. Albarede, Geochim. Cosmochim. Acta, 1999, 61, 4847 CrossRef CAS.
- C. M. Marechal, P. Telouk and F. Albarede, Chem. Geol., 1999, 156, 251 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |
