Application of olefin metathesis in oleochemistry: an example of green chemistry
J. C. Mol
Institute of Molecular Chemistry, Faculty of Science, Universiteit van Amsterdam, Nieuwe Achtergracht 166, 1018 WV, Amsterdam, The Netherlands. E-mail: jcmol@science.uva.nl
First published on 1st February 2002
Abstract
The metathesis of natural oils and fats and their derivatives is a clean catalytic reaction that can be considered as an example of green chemistry. Using this reaction, oleochemical feedstocks can be converted into valuable chemical products, directly or in only a few reaction steps. With the development of catalysts that are active and highly selective under mild reaction conditions, there are favourable perspectives for the application of the metathesis reaction in the oleochemical industry.
Green ContextThis century will see an increasing use of renewable natural resources both in the energy and chemical industries. New and improved chemistry will often be required to transform natural raw materials into useful products. Natural oils are an important source of raw materials for the oleochemicals industry and so far 14% of the world production of fats and oils are used for this purpose. In this paper olefin metathesis reactions are used to transform fats and oils via a clean catalytic route.JHC |
1. Introduction
In the twenty-first century, the availability of fossil organic feedstocks—both as energy sources and for the production of organic chemical raw materials—will gradually decrease. At the same time, the world population will grow, with an increasing demand for a higher average standard of living. Hence it is important to look for alternative feedstocks. These can be found in renewable natural resources, both for energy purposes as well as for raw materials for the chemical industry. In the latter case, much attention has already been given to making products from natural oils and fats in the oleochemical industry. Unlike petrochemicals, oleochemicals are derived from renewable resources, have good biodegradability and no net CO2 production. Therefore, the use of renewable resources is an important component of green chemistry.Natural fats and oils (composed predominantly of glyceryl esters of fatty acids) are important sources both for nutrition and as raw materials in the oleochemical industry. About 14% of the world production of fats and oils (annual production 103 million tonnes) is used in the oleochemical industry as starting material for a wide range of chemical products.1 The most important are the long-chain vegetable oils, such as soybean oil, the new high-oleic-acid varieties of sunflower seed oil and rapeseed oil (all containing mainly unsaturated C18 fatty acids), and palm oil (containing both C16 and unsaturated C18 chains). Short- and medium-chain vegetable oils, such as coconut and palm kernel oil, consist mainly of lauric (C12) and myristic (C14) acid chains and are important sources for the production of cosmetics, detergents, soaps, emulsifiers, etc. Some other vegetable oils are the source of oleochemicals on a smaller scale. An example is castor oil (consisting of 85–95% ricinoleic acid, i.e., 12-hydroxyoctadecenoic acid) that has a wide range of industrial uses. Animal fats (making up 19% of total commodity oils and fats1), such as tallow (a by-product of the meatpacking industry, containing 40% oleic acid) and lard, are also in demand as raw material, mainly because of their low price.
Fatty acid monoesters are usually obtained from the transesterification of natural oils and fats with a lower alcohol, e.g., methanol, along with glycerol. More than 90% of all oleochemical reactions (conversion into fatty alcohols and fatty amines) of fatty acid esters is carried out at the carboxy function.2 However, transformations by reactions of the carbon–carbon double bond, such as hydrogenation, epoxidation, ozonolysis and dimerization, are becoming increasingly important industrially. Here we will discuss another reaction of the carbon–carbon double bond, the olefin metathesis reaction.
Olefin metathesis is an important catalytic reaction in organic synthesis, in which olefins are converted into new products via the rupture and reformation of carbon–carbon double bonds;3eqn. (1).
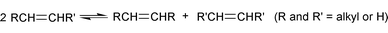 | (1) |
The key step in this process is the reaction between an olefin and a transition metal alkylidene (carbene) complex in a 2 + 2 fashion to generate an unstable metallacyclobutane intermediate. This intermediate can either revert to the starting material or open productively to afford a new metal carbene and produce a new olefin; eqn. (2). If this process is repeated often enough, eventually an equilibrium mixture of olefins will be obtained.
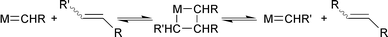 | (2) |
At the moment there are several applications of olefin metathesis in the petrochemical industry, such as the Phillips triolefin process, which produces polymerization-grade propene by cross-metathesis of ethene and 2-butene, and a process for the production of neohexene, an intermediate in the synthesis of musk perfume. A large-scale industrial process incorporating olefin metathesis is the Shell Higher Olefins Process (SHOP) for converting ethene to detergent-range alkenes. Moreover, several interesting polymeric materials are commercially produced via the so-called ring-opening metathesis polymerization of different types of unsaturated cyclic monomers, including cyclooctene, norbornene and dicyclopentadiene.3
Unsaturated fatty acid esters and oils are very promising renewable and cheap feedstocks for metathesis, which makes the metathesis reaction of interest to the chemical industry by offering novel routes to new and existing products.
2. Metathesis of unsaturated fatty acid esters
Metathesis reactions of unsaturated fatty acid esters provide a convenient, environmentally benign and highly selective route to various chemical products, directly or in only a few reaction steps. Two main types of metathesis reactions are plausible: self-metathesis (between two identical olefin molecules) and cross-metathesis (between two different olefin molecules).2.1. Self-metathesis
The first successful metathesis conversion in this area was the selective transformation by Boelhouwer and co-workers4 of methyl oleate (methyl cis-9-octadecenoate), a readily accessible unsaturated ester of oleic acid, into equimolar amounts of 9-octadecene and dimethyl 9-octadecene-1,18-dioate; eqn. (3).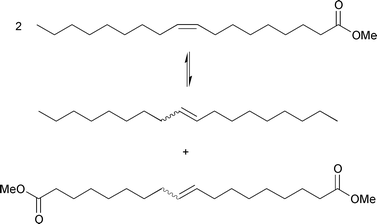 | (3) |
Using 1–2 mol% of a WCl6/Me4Sn catalyst system, equilibrium conversion was reached within 4 h at 70 °C. Because the free energy change in this type of reaction is virtually zero, the result at equilibrium is a random distribution of the alkylidene groups. Thus, starting with methyl oleate, the equilibrium mixture consists of 50 mol% of the starting material and 25 mol% of each of the two reaction products. As metathesis also brings about cis–trans isomerization (‘non-productive’ metathesis), the products as well as the starting material end up in their thermodynamic trans/cis equilibrium ratio, in which trans is favoured.
The above reaction demonstrates that, in the presence of a suitable catalyst, the metathesis of unsaturated fatty acid esters provides a convenient and selective route to unsaturated diesters and internal alkenes, both of which are useful intermediates in product synthesis. In other words, the metathesis of methyl oleate is characterized by a quantitative catalytic reaction with no by-products; i.e., it is a 100% atom-efficient process.
Unsaturated diesters can be used for the production of useful chemical products such as macrocyclic compounds. For instance, the diester obtained by metathesis of ethyl oleate has been subjected to a Dieckmann condensation, followed by hydrolysis and decarboxylation, to give a cis–trans mixture of the unsaturated macrocyclic ketone 9-cycloheptadecen-1-one (1), whose cis form, civetone, is an attractive ingredient in musk perfumes, which otherwise can only be synthesized involving many steps giving poor yields;5,6eqn. (4).
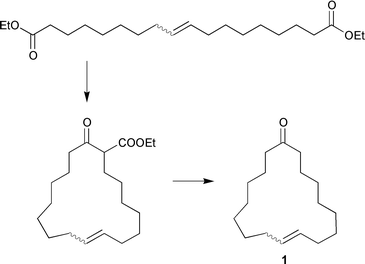 | (4) |
Moreover, unsaturated dicarboxylic esters and acids are interesting starting materials for the manufacture of unsaturated (vulcanizable) polyesters and polyamides.7 On the other hand, the co-product 9-octadecene can, for example, be dimerized and hydrogenated to give 10,11-dioctyleicosane, a lube-oil-range hydrocarbon intermediate.8
Several catalyst systems, homogeneous as well as heterogeneous, have been developed since this first example for the metathesis of unsaturated fatty acid esters and related compounds; see Section 4.
For the metathesis of methyl oleate, a very pure substrate is required. We have demonstrated a convenient new synthetic route to civetone. In this route, methyl oleate is first converted via a Claisen condensation to the doubly unsaturated ketone oleon (9,26-pentatriacontadien-18-one) (2), which can be separated in pure form from the reaction mixture. Oleon is then converted into 9-octadecene (3) and a cis–trans mixture of 9-cycloheptadecen-1-one (1) via a ring-closing metathesis reaction; eqn. (5).
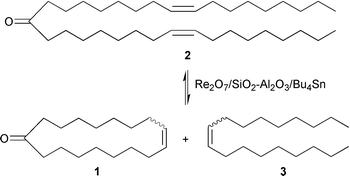 | (5) |
This reaction could be carried out at room temperature in the presence of a heterogeneous Re2O7-based catalyst.9 Although the initial results gave a low overall yield, the development of new ruthenium metathesis catalysts (see Section 4) makes the ring-closing metathesis route to macrocyclic musks very promising, despite the fact that high dilution conditions are required to reduce the possibility of intermolecular metathesis reactions between oleon molecules.
Many other unsaturated fatty acid methyl esters of the general formula Me(CH2)nCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)mCOOMe have been shown to undergo metathesis with high selectivity, such as methyl palmitoleate (methyl cis-9-hexadecenoate, n = 5, m = 7), methyl erucate (methyl cis-13-docosenoate, n = 7, m = 11), methyl petroselenate (methyl cis-6-octadecenoate, n = 10, m = 4) and methyl elaidate (the trans isomer of methyl oleate).10
CH(CH2)mCOOMe have been shown to undergo metathesis with high selectivity, such as methyl palmitoleate (methyl cis-9-hexadecenoate, n = 5, m = 7), methyl erucate (methyl cis-13-docosenoate, n = 7, m = 11), methyl petroselenate (methyl cis-6-octadecenoate, n = 10, m = 4) and methyl elaidate (the trans isomer of methyl oleate).10
Another example is the metathesis of ω-unsaturated fatty acid esters leading to an internally unsaturated long-chain dicarboxylic acid ester, e.g., the metathesis of methyl 10-undecenoate, which can be obtained by pyrolysis of methyl ricinoleate (source e.g., castor oil). The metathesis of methyl 10-undecenoate proceeds to completion when the volatile co-product ethene is removed during the reaction; eqn. (6).
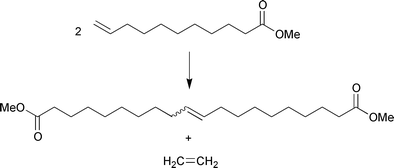 | (6) |
Metathesis of esters of polyunsaturated fatty acids, such as methyl linoleate (methyl cis,cis-9,12-octadecadienoate) and methyl linolenate (methyl cis,cis,cis-9,12,15-octadecatrienoate) leads to a variety of reaction products. Three main sets of linear products are formed: polyenes, monocarboxylic esters and dicarboxylic esters. The product distribution at equilibrium corresponds to a random scrambling of alkylidene and carboxy-alkylidene moieties leading to a 1∶2∶1 molar ratio of the three main types of reaction products. Moreover, 1,4-cyclohexadiene and cyclopolyenes are also formed, as a result of secondary intramolecular metathesis reactions,11 as illustrated for methyl linolenate in eqn. (7).
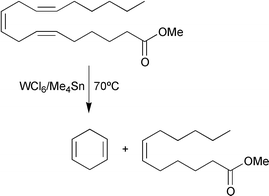 | (7) |
2.2. Cross-metathesis
Cross-metathesis of unsaturated fatty acid esters with a normal alkene is an elegant way of synthesizing more desirable homologues of these esters, and greatly extends the versatility of the metathesis reaction in the field of oleochemistry. From most industrial vegetable oil crops, predominantly fatty esters are obtained with chain lengths of 16–22 carbon atoms. These long-chain fatty acid esters can be shortened via cross-metathesis with a lower olefin to form less abundant medium-chain fatty acid esters, such as the highly-demanded detergent-range C12–C14 acid esters. An example is the cross-metathesis of methyl oleate with 3-hexene, giving 3-dodecene and methyl 9-dodecenoate; eqn. (8) .12–14 A large excess of 3-hexene can force the reaction to the product side and suppress self-metathesis of the ester.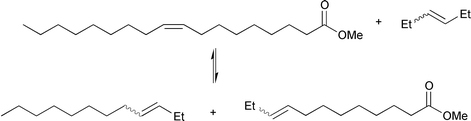 | (8) |
With regard to their chain length, these middle-chain esters are quite similar to those derived from palm kernel and coconut oils. In addition, the C11–C13 alkene co-products, with the double bond near the end of the chain, can be transformed into useful linear C12–C14 alcohols by hydroformylation, or transformed into linear alkylbenzene sulfonates.
Instead of shortening the carbon chain of unsaturated esters, it is also possible to lengthen it, as illustrated for the cross-metathesis between methyl 10-undecenoate and 3-hexene in eqn. (9).
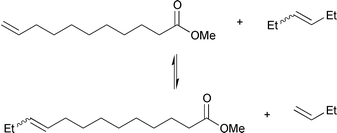 | (9) |
From a synthetic point of view, cross-metathesis reactions are very useful for the production of fine chemicals, which often are difficult to obtain by other means, or only after many reaction steps. An example is the synthesis of 1-triacontanol, CH3(CH2)28CH2OH, a plant growth stimulant. This synthesis was performed in a relatively simple two-step process by cross-metathesis of methyl erucate with 1-octadecene in the presence of a WCl6/Me4Sn catalyst, eqn. (10), followed by hydrogenation of the methyl 13-triacontenoate over a Cu/Zn catalyst at 280 °C and 250 bar.15 However, in addition to the desired metathesis products of eqn. (10), the self-metathesis products of the starting materials as well as the two ‘reverse’ cross-metathesis products were also formed.
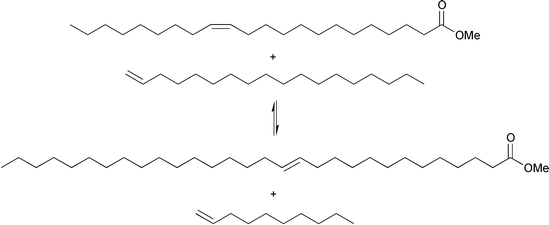 | (10) |
The synthesis of biologically active compounds such as insect pheromones is another example of organic synthesis via cross-metathesis. The use of such pheromones offers an effective, environmentally benign and selective pest control method. Thus, cross-metathesis of ethyl oleate with 5-decene gives a cis–trans mixture of ethyl 9-tetradecenoate, an insect pheromone precursor.16 Cross-metathesis of methyl cis-5-eicosenoate (obtained from meadowfoam oil) with excess 5-decene gave methyl trans-5-decenoate, which was transformed into an 83∶17 mixture of trans-5-decenyl acetate and trans-5-decenol, the sex pheromone of the peach twig borer moth, a major pest in stone fruit orchards. The mixture was active in disrupting the mating cycle of the insects.17 Other examples of organic synthesis via cross-metathesis are summarized elsewhere.10,18,19
Cross-metathesis of an unsaturated ester with a cyclic olefin leads to long-chain linear di-unsaturated esters. Thus, 1-triacontanol was also obtained by cross-metathesis between methyl oleate and cyclododecene, eqn. (11), followed by hydrogenation of the unsaturated ester product.20
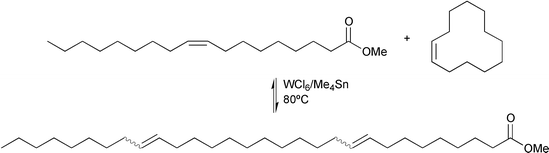 | (11) |
2.3. Ethenolysis
Cross-metathesis of an olefinic compound with ethene is denoted ethenolysis. Ethenolysis of unsaturated fatty acid esters allows the synthesis of shorter-chain ω-unsaturated esters, which have a wide range of applications. Excess ethene can easily be applied (e.g., by using elevated ethene pressures) to suppress self-metathesis of the ester and to force the conversion to completion. Ethenolysis of methyl oleate produces methyl 9-decenoate and 1-decene;21,22eqn. (12).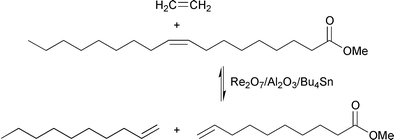 | (12) |
Methyl 9-decenoate is the key intermediate in the synthesis of many chemical products. After hydrolysis and hydrogenation, it yields decanoic acid or decanol, substances used in the synthesis of lubricants and plasticizers. It can also be used to produce fragrances (e.g., 9-decen-1-ol), pheromones (e.g., 9-oxo-trans-2-decenoic acid, a honeybee pheromone, the queen substance), prostaglandins (e.g., 9-oxodecanoic acid, a prostaglandin intermediate) etc., which are easily isolated in pure form.18,19 Methyl 9-decenoate is also the hypothetical source of many polymers and copolymers and can, for example, be converted into 10-aminodecanoic acid and then used for the production of nylon-10. Unsaturated polyesters were recently synthesized starting from some ω-unsaturated fatty acid esters that were obtained via ethenolysis of natural internally-unsaturated fatty acid methyl esters.23
Dimerization of methyl 9-decenoate to give 1,18-nonadecadien-10-one, followed by ring-closing metathesis under liberation of ethene, is another interesting route to give the macrocyclic musk civetone;9eqn. (13). With a rhenium-based catalyst, however, a low yield of 9-cycloheptadecen-1-one (trans/cis ratio 1∶1) was obtained.
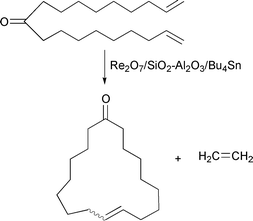 | (13) |
The same principle was recently applied using a ruthenium catalyst for the metathesis step. Dimerization of methyl 9-decenoate via a Ti-Claisen condensation, followed by ring-closing metathesis, gave a 17-membered ß-ketoester, which, after hydrolysis and decarboxylation, gave 9-cycloheptadecen-1-one. The overall isolated yield was 74% with the product having a trans/cis ratio of 3∶1.24 Unfortunately, in this type of synthesis there is little selectivity with respect to the configuration of the carbon–carbon double bond in the resulting product.
The co-product 1-decene of eqn. (12) is an important intermediate in organic syntheses, and has a variety of end uses in polymers, surfactants and lubricants.
Ethenolysis of methyl erucate (the main component of high-erucic rapeseed oil and of Crambe oil) gives another ω-unsaturated ester, methyl 13-tetradecenoate, which potentially has applications that are analogous to those of methyl 9-decenoate.
Ethenolysis of polyunsaturated fatty acid esters at elevated ethene pressure leads to good yields of 1-heptene or 1-butene (from linoleic or linolenic esters, respectively), 1,4-pentadiene, 1,4-decadiene, methyl 9-decenoate and methyl 9,12-tridecadienoate. This reaction is illustrated for methyl linoleate in eqn. (14).25
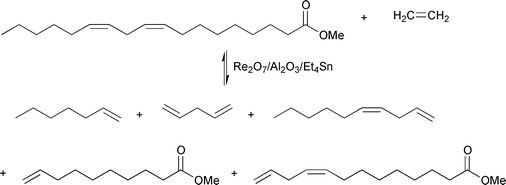 | (14) |
3. Metathesis of natural oils and fats
A variety of unsaturated fatty oils may serve as easily obtainable and relatively inexpensive raw materials for the metathesis reaction. Metathesis of fatty oils that contain triglycerides of unsaturated long-chain fatty acids proceeds intramolecularly as well as intermolecularly, the latter reaction strongly predominating. Thus, in the presence of the catalyst system WCl6/Me4Sn, olive oil, which consists mainly of glyceryl trioleate (triolein), yields 9-octadecene and polymeric triglycerides (principally dimers and trimers);19,26eqn. (15).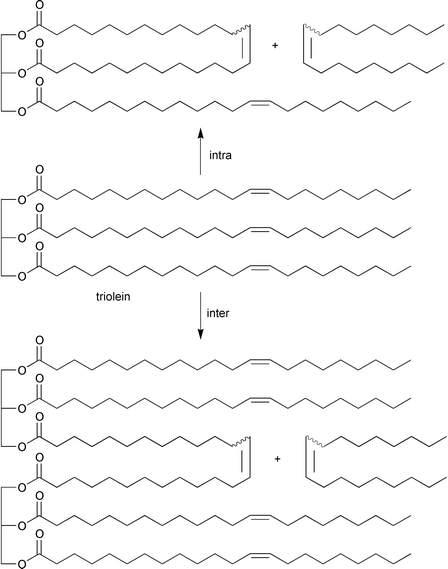 | (15) |
Both drying and semidrying oils (such as linseed oil and soybean oil, respectively) are valuable raw materials for the manufacture of oil-based paint, printing ink, synthetic resins etc.27 Metathesis of these oils (containing triglycerides of oleic, linoleic and linolenic acid) results in high-molecular-weight oils, so-called stand oils (i.e., drying oils of increased viscosity). This process of molecular enlargement does not involve the consumption of double bonds. Therefore, these high-molecular-weight oils have drying properties that are more pronounced than those of thermally polymerized oils where the polymerization process has already considerably reduced the number of double bonds available for cross-linking during the drying process.19 Thus, soybean oil that was metathesized in the presence of the WCl6/Me4Sn catalyst system and used as an additive at low concentrations dramatically
decreased the drying time of soybean oil, of benefit for printing ink vehicles, etc.28 The metathesis of a number of unsaturated vegetable oils in the presence of the ruthenium catalyst Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)Cl2(PCy3)2 was recently investigated.29
CHPh)Cl2(PCy3)2 was recently investigated.29
Cross-metathesis of distinct fatty oils (olive, soybean, rapeseed, cottonseed) consisting of long-chain unsaturated fatty acid triglycerides, with lower olefins allows, in principle, the transformation of natural fatty oils into fatty oils of lower molecular weight. An example is the production of tricaprin from olive oil by ethenolysis and subsequent hydrogenation; eqn. (16). As valuable co-products, corresponding amounts of terminal olefins are produced.
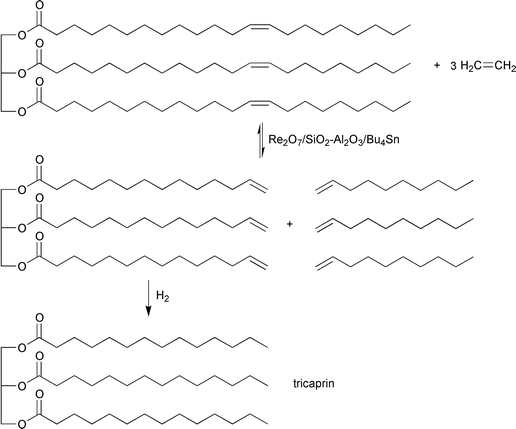 | (16) |
The ethenolysis of triolein into tridecenylglycerol proceeds, in the presence of a heterogeneous Re2O7-based catalyst, with high conversion at room temperature and an ethene pressure of 30 bar.10
4. Catalysts
Table 1 gives several examples of catalyst systems that are able to bring about the metathesis of oleic acid esters. The values reported for the turnover number (TON) are the effective TONs, this being the total number of substrate molecules converted to metathesis products per molecule of catalyst. Table 2 gives several examples of catalyst systems used for the metathesis of methyl 10-undecenoate. It follows that these metathesis reactions can proceed at room temperature. Moreover, although nearly all reactions mentioned in the Tables were performed in a solvent, a solvent is not always necessary; when a solvent is advantageous, an environmentally benign solvent such as pentane or heptane can be employed. The catalysts mentioned are also suited for cross-metathesis and ethenolysis reactions.| Catalyst | Ester/metal atoma | T/°C | tb/h | TONc | Ref. |
|---|---|---|---|---|---|
| a Molar ratio.b t = Time to reach the highest conversion.c TON = Moles of substrate converted per mol of W, Ru, Re or Mo into reaction products.d Ar = C6H3Ph2-2,6; Np = CH2CMe3.e No solvent.f Silica–alumina containing ∼25wt% Al2O3.g Silica–alumina containing 60wt% Al2O3. | |||||
| Homogeneous systems | |||||
| WCl6/Me4Sn | 75 | 110 | 2 | 38 | 26 |
| W(OC6H3Cl2-2,6)2Cl4/Bu4Pb | 50 | 85 | 0.5 | 25 | 30 |
W(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCMe3)NpCl(OAr)2(OEt2)d CHCMe3)NpCl(OAr)2(OEt2)d | 100 | 85 | 1 | 32 | 31 |
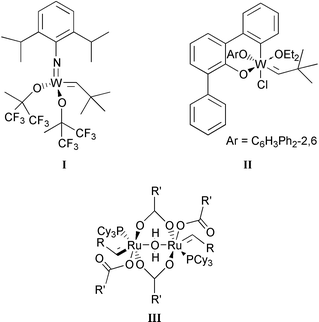
[W]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCMe3 (see formula I) CHCMe3 (see formula I) | 300 | 25 | 2–3 | 150 | 32 |
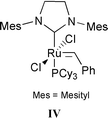
[W]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCMe3 (see formula II) CHCMe3 (see formula II) | 500 | 25 | 1 | 250 | 33 |
Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2)Cl2(PCy3)2 CPh2)Cl2(PCy3)2 | 2000 | 20 | 96 | 960 | 34 |
Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)Cl2(PCy3)2 CHPh)Cl2(PCy3)2 | 5500 | 20 | 48 | 2500 | 35 |
[Ru2]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh (III, R′ CHPh (III, R′
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF3) CF3) | 550 | 40 | 1 | 225 | 36 |
Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)Cl2(H2IMes)(PCy3) (IV)e CHPh)Cl2(H2IMes)(PCy3) (IV)e | 987![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 55 | 6 | 440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 48 |
| Heterogeneous systems | |||||
| Re2O7/Al2O3/Et4Sn | 60 | 20 | 2 | 3 | 18 |
| Re2O7/MoO3/Al2O3/Et4Sn | 60 | 20 | 2 | 30 | 18 |
| Re2O7/B2O3/Al2O3/Bu4Sn | 120 | 20 | 2 | 50 | 37 |
| Re2O7/SiO2–Al2O3/Bu4Sn | 240 | 40 | 2 | 120 | 10 |
| Re2O7/B2O3/SiO2–Al2O3/Bu4Snf | 480 | 20 | 2 | 160 | 38 |
| Re2O7/B2O3/SiO2–Al2O3/Bu4Sng | 200 | 80 | 2 | 90 | 39 |
| CH3ReO3/SiO2–Al2O3 | 100 | 25 | 2 | 27 | 40 |
| MoO3/SiO2/(CO, hν)/cyclopropane | 250 | 50 | 0.17 | 125 | 41 |
| MoO3/SiO2/(CO, laser)/cyclopropane | 1250 | 40 | 3 | 500 | 10 |
4.1. Homogeneous catalyst systems
Homogeneous metathesis catalysts generally consist of (i) a combination of a transition metal halide or oxo–halide with an alkylating co-catalyst; or (ii) a well-defined metal alkylidene (carbene) complex of a transition metal, such as Ru, Mo or W. In the former case (i.e., the classical catalysts), the active alkylidene species is generated from the alkyl groups on the co-catalyst, most probably via a double alkylation of the metal centre, followed by α-H-elimination. Functional groups, such as ester groups, in the substrate can, however, interfere with catalytic activity: they may bind to the active metal centre in competition with the carbon–carbon double bond and deactivate the catalyst, or they may react directly with the metal centre and destroy the active species.10 Therefore, not all catalyst systems that are active for the metathesis of normal olefins are active for the metathesis of functionalized olefins.Two-component catalyst systems, mainly WOCl4 and WCl6 in combination with a suitable co-catalyst, have been widely employed on a laboratory scale. These were the first catalyst systems developed for the metathesis of unsaturated fatty acid esters.3,4 They are more sensitive to moisture and air, but less expensive and easier to handle than the metal–alkylidene complexes. However, their turnover numbers are low and a toxic co-catalyst is required.
First examples of metal–alkylidene catalysts that are active for metathesis of functionalized olefins were the complexes I32 and II.33 The bulkiness of imido and aryloxide ligands prevents, to some extent, the co-ordination of the functional group to the tungsten atom and probably slows down dimerization of these electron-deficient organometallic complexes to inactive complexes.44 Next, attention was focused on ruthenium–alkylidene complexes. The well-defined ruthenium benzylidene complexes Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH
CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2)Cl2(PCy3)245 and Ru(
CPh2)Cl2(PCy3)245 and Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)Cl2(PCy3)2,46 developed by Grubbs and co-workers, are excellent functional-group-tolerant metathesis catalysts. Using the latter, we obtained 40% conversion of methyl oleate
in 4 h at a substrate/catalyst molar ratio of 550, in dichloromethane, with 96% selectivity, while with a higher substrate/catalyst ratio a TON as high as 2500 was obtained (see Table 1).35 An advantage of homogeneous catalysts is that they can easily be modified by ligand design.
CHPh)Cl2(PCy3)2,46 developed by Grubbs and co-workers, are excellent functional-group-tolerant metathesis catalysts. Using the latter, we obtained 40% conversion of methyl oleate
in 4 h at a substrate/catalyst molar ratio of 550, in dichloromethane, with 96% selectivity, while with a higher substrate/catalyst ratio a TON as high as 2500 was obtained (see Table 1).35 An advantage of homogeneous catalysts is that they can easily be modified by ligand design.
One aspect that is of interest is the stereoselective production of cis or trans isomers, e.g., in the metathesis of methyl oleate where the cis isomer of the diester can be the desired product for further conversion to fine chemicals. We have recently synthesized a new family of dimeric ruthenium carbene complexes of the general formula Ru2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHR)2(R′CO2)2(μ-R′CO2)2(PCy3)2(μ-H2O) (III) (R = Ph or –CH
CHR)2(R′CO2)2(μ-R′CO2)2(PCy3)2(μ-H2O) (III) (R = Ph or –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2; R′ = CF3, C2F5) and these are active catalysts for the metathesis of methyl oleate36 (Table 1). These systems show a very high selectivity (>99.9%) as well as an increased product stereoselectivity compared with Ru(
CPh2; R′ = CF3, C2F5) and these are active catalysts for the metathesis of methyl oleate36 (Table 1). These systems show a very high selectivity (>99.9%) as well as an increased product stereoselectivity compared with Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)Cl2(PCy3)2.
CHPh)Cl2(PCy3)2.
Recently, we obtained exciting results with a Grubbs ruthenium–carbene catalyst containing an N-heterocyclic carbene ligand, Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)Cl2(H2IMes)(PCy3) (IV; H2IMes = 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene).47 When using this complex as catalyst for the metathesis of methyl oleate, we obtained an extremely high TON of 440
CHPh)Cl2(H2IMes)(PCy3) (IV; H2IMes = 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene).47 When using this complex as catalyst for the metathesis of methyl oleate, we obtained an extremely high TON of 440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and high selectivity at a reaction temperature of 55 °C after 6 h in the absence of a solvent.48 Moreover, also for the metathesis of methyl 10-undecenoate, a very high TON of 11
000 and high selectivity at a reaction temperature of 55 °C after 6 h in the absence of a solvent.48 Moreover, also for the metathesis of methyl 10-undecenoate, a very high TON of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 was obtained. These figures justify industrial applications.†
200 was obtained. These figures justify industrial applications.†
Presently, attention has also focused on immobilizing homogeneous catalysts on a solid support. Immobilization of the ruthenium carbene complex Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)Cl2(PCy3)2 on a functionalized polystyrene support yielded a catalyst that was moderately active in the metathesis of methyl oleate.50
CHPh)Cl2(PCy3)2 on a functionalized polystyrene support yielded a catalyst that was moderately active in the metathesis of methyl oleate.50
4.2. Heterogeneous catalysts
In general, heterogeneous metathesis catalysts have the advantage that they are more favourable with respect to the separation of the catalyst from the reaction products, catalyst reuse and application in continuous processes.For the metathesis of normal olefins, heterogeneous catalysts generally consist of a transition metal oxide or chloride supported on a high-surface-area inorganic oxide. Examples are Re2O7/Al2O3, MoO3/SiO2 and WO3/SiO2. These catalysts do not contain an alkylidene moiety in their original co-ordination sphere; the initiating metal carbene is formed from the catalyst and the olefin itself. A supported Re2O7 catalyst is particularly attractive because it is active and highly selective under mild reaction conditions (20–100 °C) and can be recovered easily. Unfortunately, these catalysts show no activity for the metathesis of functionalized olefins such as unsaturated acid esters. Therefore, a major step forward was the discovery that the metathesis of unsaturated acid esters can be brought about by the catalyst Re2O7/Al2O3 when promoted with a Me4Sn co-catalyst.51
Because the tin and lead compounds are toxic, attention has been given to promoters other than tetraalkyl-tin or -lead, viz. environmentally more benign alkyl-silyl and -germanium compounds. An example of such an active promoter is Bu4Ge.56
The catalytic activity of (promoted) Re2O7/Al2O3 increases with increasing rhenium loading up to a loading of 18 wt% Re2O7. A positive correlation between the catalytic activity and the surface acidity (mainly the Brønsted acidity) has been observed.57,58 The increasing activity of Re2O7/Al2O3 catalysts can be explained on the basis of the activity of the surface ReO4 groups that have reacted with Lewis acid sites on the alumina and with the different aluminium-bonded OH groups during the preparation of the catalyst.55 The most active sites arise from the reaction between ReO4− ions and the most acidic OH groups on the alumina surface, to form Al-bonded ReO4 species. Such reactions are favoured only after the basic and neutral OH groups on the alumina have reacted to some extent.
On the other hand, when SiO2–Al2O3 is used as the catalyst support, the specific catalytic activity decreases with increasing rhenium loading.59 A high activity is obtained for a SiO2–Al2O3 supported catalyst with an alumina content of about 25 wt%, which has a high Brønsted acidity. This Brønsted acidity is due to two types of hydroxyl groups: hydroxyl groups attached to a Si atom and bridging hydroxyl groups attached to both a Si and an Al atom. At low rhenium loading ReO4–
ions react preferentially with the bridging surface hydroxyl groups during calcination, resulting in electron-poor rhenium centres (ReO4− tetrahedra), the active site precursors. This might explain why Re2O7 supported on SiO2–Al2O3 is already very active at low rhenium loading.
At higher rhenium loading, the hydroxyl groups attached to a Si atom are also replaced, resulting in inactive rhenium centres of the type ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–ReO3 (or rhenium clusters).
Si–O–ReO3 (or rhenium clusters).
Likewise, the positive effect on the activity of the addition of other metal oxides, borate or phosphate, has been attributed to an increase of the Brønsted acidity of both the supports and their corresponding catalysts.
Rhenium-based systems are only active for the metathesis of functionalized olefins when promoted with an alkylating co-catalyst. The active catalytic sites generated by a R4M (M = Sn, Pb, Ge, Si) co-catalyst may be intrinsically different from those present on the unpromoted catalyst. Based on 119Sn Mössbauer and 13C and 119Sn MAS NMR experiments with the catalyst system Re2O7/SiO2–Al2O3/R4Sn, it has been proposed that these active sites are formed via a double alkylation involving one rhenium site, followed by α-H-abstraction leading to a rhenium–alkylidene species.60,61
The molybdenum-based catalysts deactivate faster than the rhenium-based ones. Studies concerning the stability of the catalyst during continuous metathesis of propene showed a loss of activity due to an intrinsic deactivation mechanism.65
Although rhenium is a precious metal, rhenium-based catalysts are preferable to molybdenum ones because their activation is simpler and regenerability is much better.
5. Perspectives
The metathesis of natural fats and oils and their derivatives is a clean catalytic reaction that can be considered as an example of green chemistry. Starting with renewable raw materials, valuable chemical products are directly, or in only a few reaction steps, obtainable with high selectivity under mild reaction conditions, while solventless operation is possible. Therefore, the metathesis reaction has good prospects as a contribution to a sustainable chemical industry provided that sufficient raw materials are available. In particular, metathesis has prospects for making products with a high added value, such as fine chemicals.The solid rhenium oxide catalyst systems developed in our laboratory are already active at room temperature, can be easily separated from the reaction mixture and can be regenerated many times. After total deactivation the rhenium can be recovered relatively easily.
Recent findings demonstrate that new well-defined homogeneous ruthenium complexes are very effective as catalysts for the metathesis of unsaturated fatty acid esters and related compounds. Although they are still expensive and decompose irreversibly during the reaction, this is compensated by high turnover numbers.
In summary, metathesis of unsaturated fatty oils and their derivatives should now seriously be considered by the oleochemical industry as an interesting process awaiting application.
Acknowledgement
I would like to thank Maarten Dinger for his help in preparing the manuscript.References
- F. Gunstone, Inform, 2000, 11, 599 Search PubMed.
- F. Gunstone, Eur. J. Lipid Sci. Technol., 2001, 103, 307 CrossRef CAS.
- K. J. Ivin and J. C. Mol, Olefin Metathesis and Metathesis Polymerization, Academic Press, London, 1997 Search PubMed.
- P. B. Van Dam, M. C. Mittelmeijer and C. Boelhouwer, J. Chem. Soc., Chem. Commun., 1972, 1221 RSC.
- Y.-M. Choo, K.-E. Ooi and I.-H. Ooi, J. Am. Oil Chem. Soc., 1994, 71, 911 Search PubMed.
- J. Tsuji and S. Hashiguchi, Tetrahedron Lett., 1980, 21, 2955 CrossRef CAS.
- J. M. Van Thiel and C. Boelhouwer, Farbe Lack, 1974, 80, 1028 Search PubMed.
- Y.-M. Choo, K.-E. Ooi, I.-H. Ooi and D. D. H. Tan, J. Am. Oil Chem. Soc., 1996, 73, 333 Search PubMed.
- M. F. C. Plugge and J. C. Mol, Synlett, 1991, 507 CrossRef CAS.
- J. C. Mol, J. Mol. Catal., 1994, 90, 185 CrossRef CAS.
- E. Verkuijlen and C. Boelhouwer, Fette, Seifen, Anstrichm., 1976, 78, 444 Search PubMed.
- E. Verkuijlen, R. J. Dirks and C. Boelhouwer, Recl. Trav. Chim. Pays-Bas, 1977, 96(11), 86 Search PubMed.
- R. H. A. Bosma, G. C. N. van den Aardweg and J. C. Mol, J. Organomet. Chem., 1983, 255, 159 CrossRef CAS.
- S. Warwel and A. Deckers, Tenside, Surfactants Deterg., 1989, 26, 252 Search PubMed.
- J. Penninger, M. Biermann and H.-J. Krouse, Fette, Seifen, Anstrichm., 1989, 85, 239 Search PubMed.
- V. I. Bykov, T. A. Butenko and E. Sh. Finkel’shtein, Izv. Akad. Nauk SSSR, Ser. Khim., 1988, 1580 Search PubMed.
- R. L. Pederson, 1997, Lecture presented at ISOM 12, St. Augustine, FL, USA.
- J. C. Mol, J. Mol. Catal., 1991, 65, 145 CrossRef CAS.
- C. Boelhouwer and J. C. Mol, Prog. Lipid Res., 1985, 242, 43.
- D. Villemin, Tetrahedron Lett., 1983, 24, 2855 CrossRef CAS.
- R. H. A. Bosma, F. van den Aardweg and J. C. Mol, J. Chem. Soc., Chem. Commun., 1981, 1132 RSC.
- M. Sibeijn and J. C. Mol, J. Mol. Catal., 1992, 76, 345 CrossRef CAS.
- S. Warwel, J. Tillach, C. Demes and M. Kunz, Macromol. Chem. Phys., 2001, 202, 1114 CrossRef CAS.
- R. Hamasaki, S. Funakoshi, T. Misaki and Y. Tanabe, Tetrahedron, 2000, 56, 7423 CrossRef CAS.
- R. H. A. Bosma, Ph.D. Thesis, 1983, Universiteit van Amsterdam, The Netherlands Search PubMed.
- P. B. Van Dam, M. C. Mittelmeijer and C. Boelhouwer, J. Am. Oil Chem. Soc., 1974, 51, 389 Search PubMed.
- S. Hellbardt and H.-P. Patzschke, in Ullmann’s Encyclopedia of Industrial Chemistry, VCH, Weinheim, 5th edn., 1987, vol. A9, p. 55 Search PubMed.
- S. Z. Erhan, M. O. Bagby and T. C. Nelsen, J. Am. Oil Chem. Soc., 1997, 74, 703 Search PubMed.
- M. D. Refvik, R. C. Larock and Q. Tian, J. Am. Oil Chem. Soc., 1999, 76, 93 Search PubMed.
- F. Quignard, M. Leconte and J.-M. Basset, J. Mol. Catal., 1986, 36, 13 CrossRef CAS.
- F. Quignard, M. Leconte and J.-M. Basset, J. Chem. Soc., Chem. Commun., 1985, 1816 RSC.
- C. J. Schaverien, J. C. Dewan and R. R. Schrock, J. Am. Chem. Soc., 1986, 108, 2771 CrossRef CAS.
- J.-L. Couturier, C. Paillet, M. Leconte, J.-M. Basset and K. Weiss, Angew. Chem., Int. Ed. Engl., 1992, 31, 628 CrossRef.
- R. H. Grubbs and S. T. Nguyen, US Pat., 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750
750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 815 , 1998 Search PubMed.
815 , 1998 Search PubMed. - W. Buchowicz and J. C. Mol, J. Mol. Catal., 1999, 148, 97 Search PubMed.
- (a) W. Buchowicz, J. C. Mol, M. Lutz and A. L. Spek, J. Organomet. Chem., 1999, 588, 205 CrossRef CAS; (b) W. Buchowicz, F. Ingold, J. C. Mol, M. Lutz and A. L. Spek, Chem. Eur. J., 2001, 7, 2842 CrossRef CAS.
- X. Xu, C. Boelhouwer, J. I. Benecke, D. Vonk and J. C. Mol, J. Chem. Soc., Faraday Trans. 1, 1986, 82, 1945 RSC.
- R. Van Zijp, MSc thesis, 1990, University of Amsterdam, The Netherlands Search PubMed.
- S. Warwel, H. G. Jägers and A. Deckers , US Pat., 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183
183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 791, 1993 Search PubMed.
791, 1993 Search PubMed. - W. A. Herrmann, W. Wagner, U. N. Flessner, U. Volkhardt and H. Komber, Angew. Chem., Int. Ed. Engl., 1991, 30, 1636 CrossRef.
- M. Yu. Berezin, V. M. Ignatov, P. S. Belov, I. V. Elev, B. N. Shelimov and V. B. Kazansky, Kinet. Katal., 1991, 32, 379 Search PubMed.
- J. Levisalles, H. Rudler, D. Cuzin and T. Rull, J. Mol. Catal., 1984, 26, 231 CrossRef CAS.
- S. Warwel, H.-G. Jägers and S. Thomas, Fat Sci. Technol., 1992, 94, 323 Search PubMed.
- F. Lefebvre, M. Leconte, S. Pagano, A. Mutch and J.-M. Basset, Polyhedron, 1995, 14, 3209 CrossRef CAS.
- S. T. Nguyen, R. H. Grubbs and J. W. Ziller, J. Am. Chem. Soc., 1993, 115, 9858 CrossRef CAS.
- P. Schwab, M. B. France, J. W. Ziller and R. H. Grubbs, Angew. Chem., Int. Ed. Engl., 1995, 34, 2039 CrossRef CAS.
- M. A. Scholl and R. H. Grubbs, Org. Lett., 1999, 1, 953 CrossRef CAS.
- M. Dinger and J. C. Mol, Adv. Synth. Catal., submitted for publication.
- C. Chapuis and D. Jacoby, Appl. Catal. A: General, 2001, 221, 93 CrossRef CAS.
- P. Nieczypor, W. Buchowicz, W. J. N. Meester, F. P. J. T. Rutjes and J. C. Mol, Tetrahedron Lett., 2001, 41, 7103 CrossRef.
- E. Verkuijlen, F. Kapteijn, J. C. Mol and C. Boelhouwer, J. Chem. Soc., Chem. Commun., 1977, 198 RSC.
- J. C. Mol, Catal. Today, 1999, 51, 289; CrossRef CAS; erratum, Catal. Today, 1999, 52, 377 Search PubMed.
- X. Xu, P. Imhoff, G. C. N. van den Aardweg and J. C. Mol, J. Chem. Soc., Chem. Commun., 1985, 273 RSC.
- X. Xu and J. C. Mol, J. Chem. Soc., Chem. Commun., 1985, 631 RSC.
- M. Sibeijn, R. Spronk, J. A. R. van Veen and J. C. Mol, Catal. Lett., 1991, 8, 201 Search PubMed.
- R. Buffon, I. J. Marochio, C. Rodella and J. C. Mol, J. Mol. Catal. A: General, 2002, to be published Search PubMed.
- A. Ellison, A. K. Coverdale and P. F. Dearing, Appl. Catal., 1983, 8, 109 CrossRef CAS.
- X. Xu, J. C. Mol and C. Boelhouwer, J. Chem. Soc., Faraday Trans. 1, 1986, 82, 2707 RSC.
- M. Sibeijn and J. C. Mol, Appl. Catal., 1991, 67, 279 CrossRef CAS.
- R. Buffon, U. Schuchardt and A. Abras, J. Chem. Soc., Faraday Trans., 1995, 91, 3511 RSC.
- R. Buffon, M. J. D. M. Jannini and A. Abras, J. Mol. Catal., 1997, 115, 173 Search PubMed.
- J. C. Mol, Catal. Lett., 1994, 23, 113 Search PubMed.
- R. Spronk, A. Andreini and J. C. Mol, J. Mol. Catal., 1991, 65, 219 CrossRef CAS.
- R. Spronk and J. C. Mol, Appl. Catal., 1991, 76, 143 CrossRef CAS.
- K. A. Vikulov, B. N. Shelimov, V. B. Kazansky and J. C. Mol, J. Mol. Catal., 1994, 90, 59 CrossRef.
Footnote |
† N.B. In a recent article it is stated that, for the fragrance and flavour industry, the utilization of noble metals such as ruthenium and/or sophisticated ligands, is only justified ‘when the turnover number is higher
than 1000 or even 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 depending on the cost of the target product’49. 000 depending on the cost of the target product’49. |
| This journal is © The Royal Society of Chemistry 2002 |