Biological reduction of perchlorate in ion exchange regenerant solutions containing high salinity and ammonium levels
Tina M. Gingras and Jacimaria R. Batista*
Department of Civil and Environmental Engineering, University of Nevada, 4505 Maryland Parkway, Las Vegas, NV 89154-4015, USA. E-mail: jaci@ce.unlv.edu; Fax: +1 702 895 3936; Tel: +1 702 895 1585
First published on 3rd January 2002
Abstract
The most promising technologies to remove perchlorate from water are ion exchange and biological reduction. Although successful, ion exchange only separates perchlorate from water; it does not eliminate it from the environment. The waste streams from these systems contain the caustic or saline regenerant solutions used in the process as well as high levels of perchlorate. Biological reduction could be used to treat the regenerant waste solutions from the ion exchange process. A treatment scheme, combining ion exchange and biodegradation, is proposed to completely remove perchlorate from the environment. Perchlorate-laden resins generate brines containing salt concentrations up to 6% or caustic solutions containing up to 0.5% ammonium. Both, high salt and ammonium hydroxide concentrations are potentially toxic to microorganisms. Therefore, the challenge of the proposed system is to find perchlorate reducing microorganisms that are effective under such stressful conditions. Preliminary results have shown that salt concentrations as low as 0.5% reduced the perchlorate biodegradation rate by 30%; salt concentrations greater than 1% decreased this rate to 40%. Although biodegradation was seen in ammonium levels of 0.4%, 0.6% and 1%, the perchlorate biodegradation rate was 90% of that at 0% ammonium hydroxide. Further research will focus on the isolation and/or acclimation of microorganisms that are able to biodegrade perchlorate under these stressful conditions.
 Tina M. Gingras Tina M. Gingras |
Introduction
Despite the success ion exchange technology has demonstrated in removing perchlorate from waters,1–3 the perchlorate-laden resins resulting from this process are a growing concern. Some resins are not easily regenerated due to the high affinity perchlorate has for them. The high costs and difficulties associated with regenerating these resins have led to disposal of spent resin through incineration.4 Conversely, resins that can be effectively regenerated produce a waste stream containing high levels of perchlorate and other anions, and the saline and caustic components used in the regenerant solution. The contaminant perchlorate is concentrated elsewhere only to be dealt with later. Regardless of regenerating or directly disposing of the spent resins, ion exchange is an incomplete technology. To eliminate perchlorate not only from water but also from the environment, a treatment scheme is proposed integrating the use of ion exchange resins for contaminant separation and biological reduction for the elimination of perchlorate from the waste solutions. Biological reduction of perchlorate transforms the contaminant into innocuous products, chloride and oxygen. This paper summarizes the current state of the knowledge on perchlorate removal by ion exchange (IX) and predicts the composition of the waste solutions produced from regenerating spent resins. Our proposed treatment system is introduced, specific research challenges outlined, preliminary data gathered and the advantages of combining these two technologies given.Although naturally occurring in nitrate deposits gathered from Chilean mines,5,6 the presence of perchlorate in the environment is usually associated with the use and manufacture of rocket fuel and explosives. These operations utilize sodium, potassium and ammonium perchlorate salts that readily dissociate in water forming the perchlorate anion (ClO4−). The high solubility of the perchlorate anion makes it very mobile in surface and groundwaters.
Clinical studies conducted during the 1960s provide most of the current knowledge on perchlorate toxicity. In the 1950s, potassium perchlorate was used to treat hyperthyroidism, a condition also known as Grave's disease. As familiarity with perchlorate use increased, dosages of perchlorate were elevated in an effort to promote healing. The increased dosages resulted in seven cases of fatal aplastic anemia from 1961 to 1966.7 Adverse effects of this contaminant target the functioning of the human thyroid gland. By interfering with the uptake of iodine by the thyroid, perchlorate inhibits the synthesis and secretion of thyroid hormones and causes a discharge of accumulated iodine in the gland.7 Since these studies involved high dosages of perchlorate, there is relatively little data on perchlorate toxicity at low concentrations and the risks posed to human health.
The data gathered from these clinical studies provide the basis for the provisional oral reference dose (RfD) set by the US Environmental Protection Agency (US EPA). This RfD is an estimate of a daily oral exposure to the human population (including sensitive subgroups) that is likely to be without appreciable risk of deleterious non-cancer health effects during a lifetime.8 In 1997, the California Department of Health Services (CDHS) used these RfD values to set a drinking water quality action level of 18 µg L−1 to protect against the adverse health effects of perchlorate exposure.8 The Nevada Department of Environmental Protection (NDEP) has also adopted this provisional level. As of July 2001, the US EPA requires the monitoring of this unregulated contaminant in public water systems; however, the reporting of results has been delayed until the US EPA's electronic reporting system is ready to accept data.9
With proposed action levels in mind, scientists began looking for ways to adequately remove this contaminant from drinking water supplies and groundwater. Several technologies are under consideration including membrane separation, IX, activated carbon adsorption, and biological reduction. The physical and chemical characteristics of perchlorate and poor performance of other technologies led researchers to connect strong-base anionic resins (SBAX), which have been successfully used for the removal of anions such as nitrate and arsenate, with this new contaminant. In this process, resins are placed in columns through which perchlorate contaminated waters are passed. Due to its large size and low hydration energy, the perchlorate anion replaces an innocuous ion (e.g. chloride), which is initially attached to the resin. This process continues until the resin has reached a capacity where sorption of perchlorate has diminished to a point where breakthrough of perchlorate at a specific concentration in the column effluent has occurred. The resin is saturated and must be regenerated for continued use or properly disposed of. Depending on the type of resin, high concentrations of a sodium chloride or a caustic solution are passed through the columns to remove the perchlorate from the resin. Successful regeneration allows continued use of the resins.
Several types of IX resins have been investigated for perchlorate removal. Both acrylic and styrenic SBAX remove perchlorate from waters to very low levels with long bed runs.1–3 Vieira3 found that complete perchlorate removal from acrylic SBAX is possible using a 12% sodium chloride (NaCl) solution. In the same study,3 the regeneration efficiency of perchlorate-laden SBAX having a styrenic matrix ranged from 19 to 43%. These SBAX resins are currently used commercially to remove perchlorate from contaminated waters; however, low regeneration efficiencies using economically unfeasible amounts of regenerant have made disposal of the resins through incineration a more cost-effective alternative than regeneration and reuse. Efforts to increase the removal efficiency of perchlorate from these spent resins have led to modifications either in the process
or in the resins themselves. Tripp and Clifford1 found that heating spent resins to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C during regeneration decreased the perchlorate separation factor by approximately 60%. The Calgon Carbon Corporation was able to achieve a reduction in the amount of regenerate waste (brine) produced through optimization of their ISEP® System used at Big Dalton.2 Later, with the development of a proprietary catalytic reactor system called a perchlorate and nitrate destruction module (PNDM),10 the Calgon Carbon Corporation claims to have reduced perchlorate and nitrate concentrations in the waste brines to undetectable levels; however, no published data is available on the performance of the PNDM system. In a study conducted by Gu et al.,11 use of a bifunctional resin proved to be five times more effective than monofunctional
resins in removing perchlorate. Effective regeneration using FeCl4− was obtained and, through a proprietary procedure, the liquid waste stream was minimized by precipitation of FeCl3 and ClO4− out of the waste solution. One drawback to using a highly selective resin over a resin more commercially available is the higher initial cost of the resin.
°C during regeneration decreased the perchlorate separation factor by approximately 60%. The Calgon Carbon Corporation was able to achieve a reduction in the amount of regenerate waste (brine) produced through optimization of their ISEP® System used at Big Dalton.2 Later, with the development of a proprietary catalytic reactor system called a perchlorate and nitrate destruction module (PNDM),10 the Calgon Carbon Corporation claims to have reduced perchlorate and nitrate concentrations in the waste brines to undetectable levels; however, no published data is available on the performance of the PNDM system. In a study conducted by Gu et al.,11 use of a bifunctional resin proved to be five times more effective than monofunctional
resins in removing perchlorate. Effective regeneration using FeCl4− was obtained and, through a proprietary procedure, the liquid waste stream was minimized by precipitation of FeCl3 and ClO4− out of the waste solution. One drawback to using a highly selective resin over a resin more commercially available is the higher initial cost of the resin.
Although much less studied, the use of weak-base ion exchange resins (WBAX) holds some promise. Weak-base styrenic resins did not perform well in testing and were unable to remove perchlorate effectively.3 However, polyacrylic WBAX appeared to have satisfactory perchlorate-removal efficiency and very high regeneration efficiencies using either a 12% NaCl solution or 1% ammonium hydroxide (NH4OH) solution.3 The use of ammonium hydroxide in the regeneration of WBAX could produce a waste stream more amenable to biological reduction than the saline regeneration solutions, making these resins potential candidates for the proposed system.
Perchlorate has a strong oxidizing potential, yet reduction is restricted kinetically, making this anion very stable. Fortunately, microorganisms are capable of producing enzymes that can overcome the high activation energy needed for perchlorate reduction. Several studies have been conducted documenting the biological reduction of perchlorate.12–22 In the biological reduction process, perchlorate is used as an electron acceptor, and is reduced to chloride when an electron donor, nutrients and minerals are provided. Perchlorate-reducing microbes live in a broad spectrum of environments, including pristine and hydrocarbon-contaminated soils, aquatic sediments, papermill waste sludges, and farm animal waste lagoons.18 Many of these microorganisms have been isolated and are summarized in Table 1.
| Type of microbe | Source | Characteristic |
|---|---|---|
| Mixed culture12,13 | Municipal sludge | Reduction rate = 12 mg ClO4− h−1 L−1 |
| Vibrio dechloraticans Cuznesove B-116814 | Municipal sludge | Single cells, size 0.8–1 × 0.5–0.4 µm, mobile with one flagellum. Reduction rate = 70 mg ClO4− (g of biomass)−1 h−1 |
| Strain GR-115 | Activated sludge | Motile, rod-shaped. Belong to the β-subdivision of the Proteobacteria according to 16S rDNA. |
| Wolinella succinogenes (HAP-1)16 | Anaerobic sewage | Sporeless, motile, strictly anaerobic colonies are clear, circular, and mucoid catalase−. Reduction rate = 221 mg ClO4− h−1 L−1 |
| Strain CKB17 | Paper mill waste | Single polar flagellum, facultative anaerobe, completely oxidizing, non-fermentative. |
| Perclace18 | Biosolids from wastewater plant | Curved rod, facultative anaerobe. Member of the β-subdivision of the Proteobacteria by its 16S rDNA analysis. Similar to strain GR-1. |
| PDX, D8, KJ, KJ3, KJ419,20 | Municipal wastewater | Rod, motile, facultative anaerobes. PDX and KJ is similar to isolate GR-1. |
| Ideonella dechloratans21 | Activated sludge | Motile rod shaped, polarly flagellated, chemo-organotrophic organism. Belong to β-subgroup of Proteobacteria. |
| Acinetobacter thermotoleranticus22 | Match factory wastewater | Coccoid cells 0.7–1.2 µm in diameter or rods/filaments up to 60 µm in length. No flagella, facultative anaerobes. |
Early research into the reduction of perchlorate concluded that this process should be linked to nitrate reductase activity23 and, in fact, the same enzyme may be used in reduction of perchlorate and nitrate.24 In 1998, Logan25 found that although most perchlorate or chlorate strains may be denitrifying facultative anaerobes, not all denitrifiers are chlorate reducers. In some studies,26 perchlorate was unaffected by the presence of nitrate and it was suggested that the enzymes involved in perchlorate reduction were not necessarily the same as those involved in nitrate reduction. Recently in 1999, Coates et al.27 revealed that not all perchlorate-reducing bacteria use nitrate, which also suggests that the chlorate reduction pathway and the nitrate reduction pathway may be unrelated.
Although the biochemical pathways of perchlorate reduction by microorganisms are not fully known, biological degradation has been researched and used commercially to remove perchlorate from waters. Many reactor types have been investigated for perchlorate removal (Table 2). The majorities of these systems are attached growth reactors using either sand or granular activated carbon and they were able to remove perchlorate to very low levels. A variety of electron donors including ethanol, methanol, acetate, hydrogen and cheese whey have been utilized in these reactors.
| Reactor type | Type of water | ClO4− influent concentration | ClO4− effluent concentration | Electron donor |
|---|---|---|---|---|
| Anaerobic tank13 | Municipal sludge | 142–424 mg L−1 | 3 mg L−1 | N/A |
| Up-flow fixed-bed reactor with diatomaceous earth pellets media28 | Rocket fuel motor washout waste stream | 500–1500 mg L−1 | <100 mg L−1 | Brewer's yeast extract |
| Laboratory-CSTR29 | Demilitarization wastewater | ∼4000–11000 mg L−1 | Variable, 0–5000 mg L−1 | Cheese whey, yeast |
| Fluidized bed reactor with 0.7 kg m−3 day−1 loading and sand and activated carbon media30 | Drinking water well | 6–7 mg L−1 | <4–40 µg L−1 | Acetate, methanol, ethanol |
| Fixed film fluidized bed with activated carbon as a medium31 | Groundwater | 40 µg L−1 | 4 µg L−1 | Acetate, hydrogen |
| Fixed film reactor packed with celite32 | Groundwater | 0.7 mg L−1 | <4 µg L−1 | Hydrogen |
| Fixed film reactor with activated carbon as media/hydrogen oxidizing reactor33 | Synthetic water | 35 mg L−1 | <4 µg L−1 | Hydrogen |
| 240 mg L−1 | 3–8 mg L−1 | Acetate | ||
| Hollow-fiber membrane-immobilized biofilm33 | Synthetic water | 1–2.5 mg L−1 | 30–50 µg L−1 | Hydrogen |
| Membrane-immobilized biofilm12 | Synthetic water | 100–1000 mg L−1 | <5 µg L−1 | Lactate |
| Up-flow bioreactor pack with sand34 | Synthetic water | 20 mg L−1 | <4 µg L−1 | Acetate |
| Autotrophic packed bed biofilm reactor35 | Synthetic water | 740 µg L−1 | 460 µg L−1 | Hydrogen |
Potential composition of IX regenerant solutions
The composition of the waste streams from the regeneration of perchlorate-laden resins will vary depending on the type of resin utilized, the removal and regeneration efficiencies of the resin, and the characteristics of the influent water. Two potential regenerants for perchlorate-laden resins are either salts or caustic solutions; sodium chloride (NaCl), ammonium hydroxide (NH4OH) and sodium hydroxide (NaOH) have been used.1,3 Typical NaCl concentrations in regenerant solutions range from 6 to 12%.3 Vieira3 used a 1% NaOH solution to regenerate the WBAX in his study. The original concentration of the regenerant will be diluted in the waste stream as a result of the displacement of the water already present in the resin at the onset of regeneration and the rinsing cycle prior to the next service cycle. Generally, 3–5 bed volumes (BV) of regenerant are needed for regeneration and 2–3 BV of water for rinsing.36 Thus, the concentration of the waste stream will be reduced to approximately 40–50% the original regenerant solution. In the case of perchlorate-laden resins, the concentration of brine waste solution would be approximately 3–6% NaCl and that of sodium or ammonium hydroxide approximately 0.5%. In addition to perchlorate, the waste solutions will contain several anions (e.g. nitrate, sulfate, bicarbonate, chlorate) and the constituents (e.g. Na+, Cl−, NH4+, OH−) of the regenerants used.IX processes transfer perchlorate to the resin and ultimately to a regenerant waste stream. Since perchlorate is easily reduced biologically, biodegradation could potentially be used to treat the regenerant waste stream, thus eliminating perchlorate from the environment. However, several challenges exist that must be overcome before perchlorate-containing IX wastes are successfully treated. The regenerant wastes may contain high salinity levels, high pH, and high ammonium levels. Only a few studies have investigated the effects of salinity on perchlorate biodegradation. Liu12 showed that perchlorate biodegradation was extremely hindered by salt concentrations as low as 1% and no biodegradation could be observed in salt concentrations above 4%. Coppola29 demonstrated that total dissolved solids concentrations ranging from 2–3% inhibited perchlorate reduction. In a recent paper,37 using microbial cultures harvested from saline environments to degrade perchlorate, Logan et al. showed that growth rates for these microorganisms are hindered by high salt concentrations. Optimum growth occurred at 5% salt concentration (0.06 day−1); at 9% and 11% salt concentration, growth rates decreased to 0.039 day−1. Perchlorate biodegradation has been observed in pH values ranging from 6 to 8.5, but very little research has been performed on the effects of pH on perchlorate biodegradation. Both ammonia (NH3) and ionized ammonium ion (NH4+) have been found to be toxic to microorganisms. Ammonium levels of 3000 mg L−1 inhibit anaerobic systems, while approximately 100 mg L−1 of ammonia are biologically toxic.38 The treatment of several wastes containing high concentrations of ammonia or ammonium has been challenging and it is still the subject of intense research.39–41
The specific objective of this paper was to consider the feasibility of integrating ion exchange and biological reduction in a treatment system to eliminate perchlorate from the environment. We present preliminary results on the effects of high salinity and ammonium levels on perchlorate biodegradation and discuss the research direction needed to make this system available as a complete technology.
Materials and methods
Preliminary testing was performed to determine the influence of NaCl and NH4OH on perchlorate biodegradation using a mixed culture of known perchlorate degraders.Microbial culture
A microbial culture (herein called the BALI culture) was enriched from a returned activated sludge sample taken from the Clark County Sanitation District (CCSD) wastewater treatment plant in Nevada. This culture was used successfully to degrade perchlorate during studies of a membrane-immobilized biofilm reactor in 1999.12 A modified version of the nutrient/minerals media devised by Van Ginkel et al.42 was used in the experiments. The pH was maintained at 7.0 by phosphate buffer. The ratio of lactate (300 mg L−1) to perchlorate (100 mg L−1) maintained in the reactor was 3∶1. The reactor was incubated anaerobically and continuously stirred at 23 ± 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. This culture was used as the inoculum to the culture tube experiments performed at different levels of salinity and ammonium.
°C. This culture was used as the inoculum to the culture tube experiments performed at different levels of salinity and ammonium.Culture tube experiments
In the culture tube testing, the tubes, caps, nutrient/minerals, buffer, lactate, and perchlorate solutions were autoclaved prior to their use in the experiments in order to avoid contamination by other microorganisms. The buffer and nutrient/mineral media were of the same composition as those used in the 4 L reactor. The microbial inoculum was the BALI culture. The autoclaved medium containing the desired amounts of nutrients, buffer, perchlorate, and lactate was transferred to 8.5 mL culture tubes. Specific concentrations of NaCl (0.5%, 1.0%, and 1.5%) and NH4OH (0.4%, 0.6%, and 1.0%) were added to the culture tubes. Prior to addition of these chemicals, the pH of the ammonium hydroxide was neutralized to 7.0 by titration with sulfuric acid. Next, the desired volume of a suspension of the BALI culture was added to each tube. The microbial concentration was measured gravimetrically by using a 0.45 µm acetate membrane filter (Osmonics Inc.). The tubes were then capped and maintained in an anaerobic hood 23 ± 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for incubation. Five replicate tubes were prepared for each perchlorate/salinity and perchlorate/ammonium hydroxide level combination. In addition to the experimental tubes, two sets of control tubes were added to the experiment; one containing no microbes (abiotic control) and another containing no lactate (electron donor control). The concentrations of perchlorate, lactate, and microbes dispensed into all tubes except for the controls were 100 mg L−1, 500 mg L−1 and 20 mg L−1 as suspended solids, respectively.
°C for incubation. Five replicate tubes were prepared for each perchlorate/salinity and perchlorate/ammonium hydroxide level combination. In addition to the experimental tubes, two sets of control tubes were added to the experiment; one containing no microbes (abiotic control) and another containing no lactate (electron donor control). The concentrations of perchlorate, lactate, and microbes dispensed into all tubes except for the controls were 100 mg L−1, 500 mg L−1 and 20 mg L−1 as suspended solids, respectively.At prescribed intervals, the increase in turbidity (absorbance) was measured directly in the tubes at 600 nm using a spectrophotometer (Spectronic 20, Bausch and Lomb, Rochester, NY). Tubes were mixed well before each measurement using a Labnet VX100 vortex mixer. Samples were collected from the culture tubes and analyzed for perchlorate using a DX-120 ion chromatograph with a Dionex IonPac AS11 4 mm (10-32) separation column and IonPac AG-11 4 mm (10-32) guard column. The eluent for this analysis consisted of a 49 mM sodium hydroxide solution.
Determination of growth coefficients
Absorbance measurements were used to estimate the growth coefficient of the perchlorate degrading microbes at different salinity and ammonium levels. Only the data corresponding to the exponential growth phase was used in the calculations. Any tubes possessing data points not comparable to the other tubes in the series were excluded from further analysis. For exponential microbial growth, the rate of growth is directly proportional to the initial number of microbes present and the growth coefficient can be calculated by plotting the logarithm of the absorbance versus time [eqn. (1)]. The slope of the line is the growth coefficient (μ).| ln A = ln A0 + μt | (1) |
Results and discussion
Composition of waters contaminated with perchlorate
In the United States, there are two types of waters contaminated with perchlorate, those with very high levels (mg L−1 range) and those with lower perchlorate levels (µg L−1 range). The latter are associated with migratory plumes of groundwater contaminated by intensive perchlorate use or manufacturing; while the former are the direct result of perchlorate use or manufacturing. The reported composition of several ground and surface waters contaminated with perchlorate are depicted in Table 3. The contaminated groundwater at Kerr McGee, Nevada,3 and the rocket manufacturing plants in California43 contain perchlorate concentrations that range from 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 3
000 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 µg L−1. Some of these waters also have higher levels of sulfate and nitrate. On the other
hand, available data from drinking water sources indicate perchlorate levels of 8–200 µg L−1 and nitrate and sulfate concentrations below the drinking water standards (250 mg L−1 and 44.3 mg L−1 as NO3−, respectively).2,8,45,46
000 µg L−1. Some of these waters also have higher levels of sulfate and nitrate. On the other
hand, available data from drinking water sources indicate perchlorate levels of 8–200 µg L−1 and nitrate and sulfate concentrations below the drinking water standards (250 mg L−1 and 44.3 mg L−1 as NO3−, respectively).2,8,45,46
| Location | ClO4−/µg L−1 | NO3−/mg L−1 | SO42−/mg L−1 | O2/mg L−1 | ClO3−/mg L−1 | Cl−/mg L−1 | pH |
|---|---|---|---|---|---|---|---|
| Edwards Air Force Base, CA43 | 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1 | 180 | 2 | — | 360 | 6.2 |
| DOD Site, WV | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 4 | 55 | — | — | 25 | 6.7 |
| Rocket Manufacturing Site, CA43 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 2 | 75 | — | — | — | — |
| Aerojet Superfund Site, CA43 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 4 | 40 | 4 | — | — | 6.8 |
| Aerojet, Sacramento, CA44 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 1.5 | 6 | — | — | — | 7.5 |
| Groundwater Wells, Redlands, CA45 | 50 | 61.2 | 14.9 | — | — | 7 | 6.9 |
| Kerr McGee Seepage, Las Vegas, NV3 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–3 000–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 | 51.7 | 2069 | — | 100 | 2077 | 7.85 |
| Big Dalton Site Water Wells, CA2 | 18–76 | 20–28 | 41–67 | — | — | 20–35 | — |
| San Gabriel Wells, CA8 | 80–200 | — | — | — | — | — | — |
| Lake Mead, NV46 | 8–20 | 1–5 | 250–410 | — | — | 85–172 | 7.3–8.6 |
Composition of waste stream from regeneration of perchlorate-laden IX resins
The composition of the IX regenerant solutions resulting from treating these two different types of perchlorate-contaminated waters will vary. Table 4 depicts the actual compositions of IX brines generated by treating waters containing both low and high levels of perchlorate. As expected, perchlorate concentrations in the brines from influent waters containing a high initial perchlorate level are much greater than those generated from lower initial perchlorate concentration waters. The salt concentration in the brines is reduced by 50% of the original brine concentration used. Although investigations have been conducted using caustic solutions to regenerate WBAX, no loading and regeneration using actual contaminated waters has been reported. Similar to what is observed for SBAX, the waste regenerant will be diluted by the rinse water and the ammonium concentrations reduced to approximately 50% of the original concentration. Thus, ammonium concentrations in this waste are expected to be around 0.5% when using a regenerant concentration of 1% NH4OH. Additionally, the pH of these solutions is expected to be high and neutralization with acid will be needed to bring the pH to values amenable to biodegradation. The presence of other anions such as nitrate and chlorate in the regenerant solutions will imply high costs of electron donor for the process, because some of these anions are preferentially biodegraded to perchlorate and they also consume electron donors.Effects of different levels of salinity and ammonium on perchlorate biodegradation
Preliminary tests have been conducted to investigate the effects of varying salt and ammonium levels on perchlorate biodegradation. At 0% NaCl addition, perchlorate biodegradation rates of greater than 11 mg day−1 were observed (Fig. 1). At 0.5% salt, perchlorate biodegradation was reduced by over 50% to 5 mg day−1. In the presence of a 1–1.5% salt concentration, a reduction of over 90% in the biodegradation rate was observed. For all ammonia levels greater than 0.4%, the perchlorate biodegradation rate decreased by more than 80%, showing the toxic effects of ammonia. In the presence of both salinity and ammonium, biodegradation of perchlorate by the BALI culture was limited, indicating that the commonly isolated perchlorate-reducing cultures are not able to biodegrade perchlorate found in IX regenerant waste solutions at acceptable rates.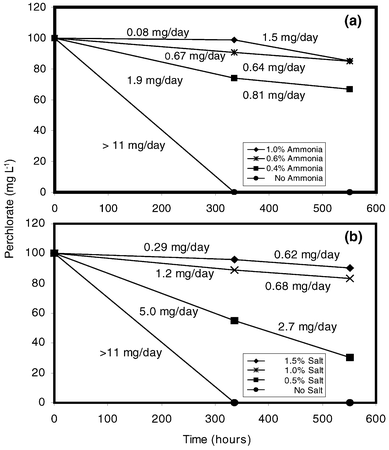 | ||
| Fig. 1 Biological reduction of perchlorate in the presence of different concentrations of ammonia (a) and salinity (b); biomass concentration = 20 mg L−1 of suspended solids. | ||
Absorbance measurements were used to estimate the growth coefficients of the perchlorate degrading microbes. Fig. 2 shows the regression for the determination of these coefficients at different ammonium and salt concentrations. The calculated growth coefficients are summarized in Table 5. The values shown in Table 5 are the average of the growth coefficients for each graph presented in Fig. 2. Notice that a 32% reduction in growth coefficient is observed at 0.5% salt as compared to the control (0% salt). More than 40% reduction is detected for salt levels greater than 1%. For ammonium, a significant reduction, of 90% or greater, was experienced for all ammonium levels tested. Although the ammonium results seem at first glance very discouraging, one must realize the potential concentration of ammonium in the regenerant wastes will be approximately 0.5%. Two of the levels tested were well above this expected concentration. In addition, biodegradation was observed, albeit at low rates, for ammonium concentrations as high as 1%. This suggests the potential to acclimate a microbial culture to biodegrade perchlorate at high ammonium levels.
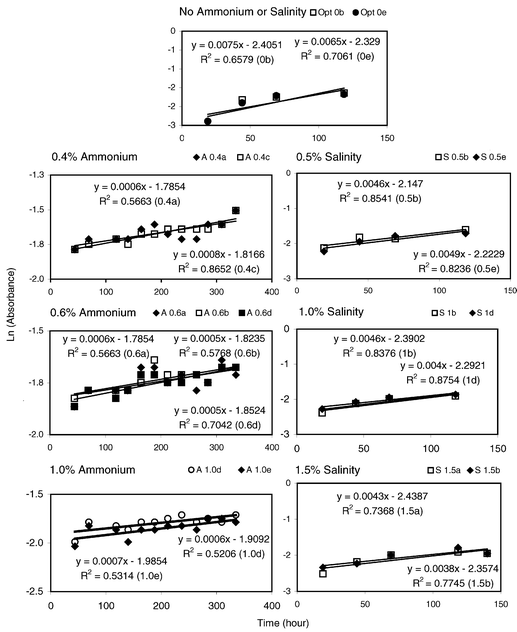 | ||
| Fig. 2 Interference of salinity and ammonium on perchlorate biodegradation. | ||
| Salt | Ammonium | ||||||
|---|---|---|---|---|---|---|---|
| Concentration (%) | µ/day−1 | R2 | Relative reduction (%) | Concentration (%) | µ/day−1 | R2 | Relative reduction (%) |
| a No additional salt or ammonium was added to the growth medium in the culture tubes. The salt and ammonium concentrations present in the growth medium were <0.02% and <0.08%, respectively. | |||||||
| 0a | 0.168 | 0.68 | 0 | 0a | 0.168 | 0.68 | 0 |
| 0.5 | 0.114 | 0.84 | 32 | 0.4 | 0.017 | 0.72 | 90 |
| 1 | 0.103 | 0.86 | 39 | 0.6 | 0.013 | 0.62 | 92 |
| 1.5 | 0.097 | 0.76 | 42 | 1 | 0.016 | 0.53 | 90 |
In summary, the preliminary results show that the mixed BALI culture was not able to biodegrade perchlorate contained in IX waste at acceptable rates. Further research will focus on acclimating and/or isolating microbial cultures that are salt-tolerant and/or able to biodegrade perchlorate at high ammonium levels. A starting point is the use of recently reported marine organisms36 that are capable of biodegrading ammonia. Efforts will also be directed towards determining the inhibitory kinetics of perchlorate degradation for different levels of salinity and ammonium.
References
- R. Tripp and D. A. Clifford, in Perchlorate in the Environment, ed. E. T. Urbansky, Plenum, New York, 2000, pp. 123–134 Search PubMed.
- Calgon Corporation, Big Dalton Perchlorate Removal Pilot Study Final Report, submitted to the Main San Gabriel Basin Water Master, Calgon Corporation, Pittsburgh, PA, October 1998 Search PubMed.
- A. Vieira, MS Thesis, University of Nevada, Las Vegas, NV, 2000.
- Nevada Department of Environmental Protection (NDEP), Permit 0023060, February 10, 1999.
- A. A. Schilt, Perchloric Acid and Perchlorates, The G. Frederick Smith Chemical Company, Columbus, OH, 1979 Search PubMed.
- S. Susarla, T. W. Collette, A. W. Garrison, N. L. Wolfe and S. C. McCutcheon, Environ. Sci. Technol., 1999, 33, 3469 CrossRef CAS.
- J. Wolff, Pharmacol. Rev., 1998, 50, 89 Search PubMed.
- EPA, http://www.epa.gov/OGWDW/ccl/perchlor/perchlo.html, 1999.
- Fed. Regist., 66, 2001, 2273 Search PubMed.
- K. R. Venkatesh, S. M. Klara, D. L. Jennings and N. J. Wagner, in Perchlorate in the Environment, ed. E. T. Urbansky, Plenum, New York, 2000, pp. 147–154 Search PubMed.
- B. Gu, G. M. Brown, S. D. Alexandratos, R. Ober, J. A. Dale and S. Plant, in Perchlorate in the Environment, ed. E. T. Urbansky, Plenum, New York, 2000, pp. 165–176 Search PubMed.
- J. Liu, MS Thesis, University of Nevada, Las Vegas, NV, 2000.
- S. V. Yakolev, J. V. Voronov, V. N. Korenkov, A. B. Nevsky, V. A. Dobrikova, T. A. Karjukhina, I. N. Churbanova and J. M. Laskov, US Pat., 3755156, 1973.
- V. N. Korenkov, V. I. Romanenko, S. I. Kuznetsov and J. V. Voronov, US Pat., 3943055, 1976.
- G. B. Rikken, A. G. M. Kroon and C. G. van Ginkel, Appl. Microbiol. Biotechnol., 1996, 45, 420 CrossRef CAS.
- H. Attaway and M. D. Smith, US Pat., 5302285, 1994.
- R. A. Bruce, L. A. Achenbach and J. D. Coates, Environ. Microbiol., 1999, 1, 319 Search PubMed.
- D. C. Herman and W. T. Frankenberger Jr, J. Environ. Qual., 1999, 28, 1018 Search PubMed.
- P.T. Mulvaney, MS Thesis, Pennsylvania State University, University Park, PA, 1999.
- B. E. Logan, Appl. Environ. Microbiol., 2001, 67, 2499 CrossRef CAS.
- A. Malmqvist, T. Welander, E. Moore, A. Ternstrom, G. Molin and I. Stenstrom, Syst. Appl. Microbiol., 1994, 17, 58 Search PubMed.
- V. Stepanyuk, G. F. Smirnova, T. M. Klyushnikova, N. I. Kanyuk, L. P. Panchenko, T. M. Nogina and V. I. Prima, Nov. Mikrobiologiya, 1993, 61, 490 Search PubMed.
- H. Attaway and M. Smith, J. Ind. Microbiol., 1993, 12, 408 Search PubMed.
- E. Hackenthal, Biochem. Pharm., 1965, 14, 1313 Search PubMed.
- B. E. Logan, Bioremediation J., 1998, 2, 69 Search PubMed.
- W. Wallace, T. Ward, A. Breen and H. Attaway, J. Ind. Microbiol., 1996, 16, 68 Search PubMed.
- J. D. Coates, U. Michaelidou, R. A. Bruce, S. M. O'Connor, J. N. Crespi and L. A. Achenbach, Appl. Environ. Microbiol., 1999, 65, 5234 CAS.
- W. Wallace, S. Beshear, D. Williams, S. Hospadar and M. Owens, J. Ind. Microbiol. Biotech., 1998, 20, 126 CrossRef CAS.
- E. N. Coppola, “ARA Perchlorate Treatment Experience”, in Proceedings of the 5th Annual Joint Services Pollution Prevention & Hazardous Waste Management Conference & Exhibition, sponsored by the Air Force Research Laboratory, San Antonio, TX, August 23–24, 2000, ed. L. Cook, Air Force Research Laboratory, Tyndall AFB, FL, 2000 Search PubMed.
- M. Greene and M. P. Pitre, in Perchlorate in the Environment, ed. E. T. Urbansky, Plenum, New York, 2000, pp. 241–257 Search PubMed.
- J. G. Catts, Proc. 218th Am. Chem. Soc. Meet., Div. Environ. Chem, 1999, 39, 107 Search PubMed.
- T. Giblin, D. Herman and W. T. Frankenberger Jr, J. Environ. Qual., 2000, 29, 1057 Search PubMed.
- B. E. Rittmann, R. Nerenberg and I. Najm, in Proceedings of the AWWA Inorganic Contaminants Workshop, American Water Works Association, Albuquerque, NM, 2000 Search PubMed.
- K. Kim and B. E. Logan, Water Res., 2001, 35, 3071 CrossRef CAS.
- J. P. Miller and B. E. Logan, Environ. Sci. Technol., 2000, 34, 3018 CrossRef CAS.
- D. A. Clifford, in Water Quality and Treatment Handbook of Community Water Supplies AWWA, ed. F. Pontius, McGraw-Hill, New York, 4th edn., 1990, pp. 561–637 Search PubMed.
- B. E. Logan, J. Wu and R. F. Unz, Water Res., 2001, 35, 3034 CrossRef CAS.
- P. L. McCarty and R. E. McKinney, J. Water Pollut. Control Fed., 1961, 33, 399 Search PubMed.
- P. Ilies and D. S. Mavinic, Environ. Technol., 2000, 22, 289 Search PubMed.
- M. Yani, M. Hirai and M. Shoda, Bioengineering, 1998, 85, 502 Search PubMed.
- N. Kim, Y. Sugano, M. Hirai and M. Shoda, J. Biosci. Bioeng., 2000, 90, 410 Search PubMed.
- C. G. van Ginkel, C. M. Plugge and C. A. Stroo, Chemosphere, 1995, 31, 4057 CrossRef CAS.
- E. N. Coppola, “ARA Perchlorate Treatment Experience”, in Proceedings of the 5th Annual Joint Services Pollution Prevention & Hazardous Waste Management Conference & Exhibition, sponsored by the Air Force Research Laboratory, San Antonio, TX, August 23–24, 2000, ed. L. Cook, Air Force Research Laboratory, Tyndall AFB, FL, 2000 Search PubMed.
- E. Cox, “In Situ Bioremediation of Perchlorate Contaminated Groundwater”, in Proceedings of the 5th Annual Joint Services Pollution Prevention & Hazardous Waste Management Conference & Exhibition, sponsored by the Air Force Research Laboratory, San Antonio, TX, August 23–24, 2000, ed. L. Cook, Air Force Research Laboratory, Tyndall AFB, FL, 2000 Search PubMed.
- B. Gu and G. M. Brown, “Highly Selective and Regenerable Anion Exchange Resins for Treatment of Perchlorate Contaminated Waters”, in Proceedings of the 5th Annual Joint Services Pollution Prevention & Hazardous Waste Management Conference & Exhibition, sponsored by the Air Force Research Laboratory, San Antonio, TX, August 23–24, 2000, ed. L. Cook, Air Force Research Laboratory, Tyndall AFB, FL, 2000 Search PubMed.
- R. Boralessa and J. R. Batista, in Proceedings of the AWWA Inorganic Contaminants Workshop, American Water Works Association, Albuquerque, NM, 2000 Search PubMed.
| This journal is © The Royal Society of Chemistry 2002 |
