Preferential adsorption of fluorescing fulvic and humic acid components on activated carbon using flow field-flow fractionation analysis
Kathryn H.
Schmit
a and
Martha J. M.
Wells
*b
aBanner Associates Inc., 409 22nd Ave. South, Brookings, SD 57006. E-mail: kathryns@bannerbkgs.com; Fax: (605)692-5714; Tel: (605)692-6342
bCenter for the Management, Utilization and Protection of Water Resources and Department of Chemistry, Tennessee Technological University, Box 5033, Cookeville, TN 38505. E-mail: mjmwells@tntech.edu; Fax: (931)372-6346; Tel: (931)372-6123
First published on 14th January 2002
Abstract
Activated carbon treatment of drinking water is used to remove natural organic matter (NOM) precursors that lead to the formation of disinfection byproducts. The innate hydrophobic nature and macromolecular size of NOM render it amenable to sorption by activated carbon. Batch equilibrium and minicolumn breakthrough adsorption studies were performed using granular activated carbon to treat NOM-contaminated water. Ultraviolet (UV) absorption spectroscopy and flow field-flow fractionation analysis using tandem diode-array and fluorescence detectors were used to monitor the activated carbon sorption of NOM. Using these techniques, it was possible to study activated carbon adsorption properties of UV absorbing, fluorescing and nonfluorescing, polyelectrolytic macromolecules fractionated from the total macromolecular and nonmacromolecular composition of NOM. Adsorption isotherms were constructed at pH 6 and pH 9. Data were described by the traditional and modified Freundlich models. Activated carbon capacity and adsorbability were compared among fractionated molecular subsets of fulvic and humic acids. Preferential adsorption (or adsorptive fractionation) of polyelectrolytic, fluorescing fulvic and humic macromolecules on activated carbon was observed. The significance of observing preferential adsorption on activated carbon of fluorescing macromolecular components relative to nonfluorescing components is that this phenomenon changes the composition of dissolved organic matter remaining in equilibrium in the aqueous phase relative to the composition that existed in the aqueous phase prior to adsorption. Likewise, it changes the composition of dissolved organic matter remaining in equilibrium in the aqueous phase relative to the adsorbed phase. This research increases our understanding of NOM interactions with activated carbon which may lead to improved methods of potable water production.
Introduction
Humic substances are heterogeneous, polydisperse forms of natural organic matter (NOM) that are ubiquitous in water and impact potable water production. The color-imparting properties of NOM historically have been considered a problem in waters used as drinking water sources. More recently, the treatment of water with chlorine and other halogen-based oxidants in the presence of NOM has been implicated in the formation of disinfection byproducts (DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs). Moreover, humic substances facilitate pollutant transport by binding organic and heavy metal contaminants.The composition and conformation of humic substances have been intensely researched; but, because of their complex nature, the primary and secondary molecular structure of these chemicals remains ill defined.1,2 Poor understanding of the relationship between NOM composition and reactivity results in severe economic pressure on the drinking water industry as they attempt to reduce levels of DBPs.
NOM is operationally considered to be composed of particulate organic matter (POM ≥ 1.0 µm), colloidal organic matter (0.22 µm ≤ COM ≤ 1.0 µm), and dissolved organic matter (DOM < 0.22 µm).3 DOM is comprised of heterogeneous organic compounds including humic and fulvic acids that are operationally defined based on solubility in acidic and basic solution. DOM may be of aquatic or terrestrial origin, and compositional differences among soil, stream, and marine sources have been reported.4 DOM is found in the microgram per liter range in groundwater and in the milligram per liter range in surface freshwater.
One treatment process used for removing NOM from water is granular activated carbon (GAC) adsorption. At concentrations as low as 3 to 4 mg L−1, humic substances interfere with the removal of synthetic organic chemicals (SOCs) such as pesticides from water treated by activated carbon adsorption.5,6 The nature of this interference is not well understood, so further characterization of humic substance–activated carbon interactions is needed.3
Following the pioneering work by Beckett et al.,7–9 research in this laboratory explores the development of flow field-flow fractionation (flow FFF) analysis for study of DOM. Diffusion coefficients and molecular weight distributions of humic and fulvic acids determined by flow FFF were previously reported from this research.10,11 Current research pursues the use of flow FFF to determine the nature of interactions between DOM and activated carbon. During this investigation,12 preferential adsorption (or adsorptive fractionation) of polyelectrolytic, fluorescing humic macromolecules on activated carbon was observed. Data to support that conclusion are presented here.
Preferential adsorption of certain humic components on activated carbon was also demonstrated by Lee13 and Kilduff et al.14 Kilduff et al.14 used high-performance size-exclusion chromatography (HPSEC) to demonstrate that small molecular-size components were adsorbed preferentially from aqueous solution by activated carbon. Kaiser and Zech15 reported the preferential sorption of a hydrophobic DOM fraction relative to a hydrophilic fraction on soils and related mineral phases, which supports the conclusion that DOM did not adsorb as a unique substance.
Lower molecular weight humic and fulvic acids have been reported to fluoresce more intensely than those of higher molecular weight.16,17 However, whether the preferentially adsorbed humic components observed by other researchers are of the same molecular character as the components observed in this research has not been determined. The corroborative results obtained by different analytical techniques in this work and that of others mutually support the premise that preferential adsorption of some humic components on activated carbon occurs.
Ultimately, researchers seek increased understanding of the relationship between NOM composition and reactivity. Preferential adsorption of humic substances on activated carbon influences the composition of the equilibrium concentration remaining in the aqueous phase. Whether this effect is beneficial or detrimental to the production of potable water or whether this observation can be exploited to optimize humic substance removal during water treatment remains to be explored.
Experimental procedures
Activated carbon adsorption methodology
Solutions of DOM used in the isotherm studies were prepared by weighing 20 mg of Aldrich humic acid (Aldrich Chemical Company) or Suwannee River fulvic acid (International Humic Substances Society) on an analytical balance to the nearest 0.01 mg and dissolving in 1 L of either pH 6 or pH 9 buffer solution. The solution was then pressure filtered through a 0.45 µm nylon filter (Fisher Scientific).
An Aldrich humic acid extract was prepared according to a procedure adapted from Summers et al.6 by dissolving 500 mg of Aldrich humic acid in 1 L of type I water and adjusting the pH above 11 using 2 N NaOH and mixing approximately for 2 h. The pH was adjusted to below 2 using concentrated o-phosphoric acid (85%). By definition, the portion of the Aldrich humic substance that remains in solution under these extraction conditions is a fulvic fraction. The resulting solution was pressure filtered through a 0.75 µm acidified glass fiber filter (Fisher Scientific) and then filtered with a 0.45 µm acidified nylon filter (Fisher Scientific). The pH was adjusted to 8 using 2 N NaOH. The concentrated extract solution was then diluted 1∶8 and buffered at a pH of 6 or 9.
The minicolumn breakthrough studies were performed using a 20 mg L−1 Aldrich humic substance solution buffered at pH 6. A concentrated stock solution was prepared by dissolving 3.6 g of Aldrich humic acid in 18 L of pH 6 buffer solution and pressure filtering through a 0.45 μm nylon filter. Sixteen liters of the filtrate were added to 144 L of pH 6 buffer in a 200 L drum. The solution was mixed for approximately 4 h using a propeller type mixer.
Dissolved organic carbon (DOC) was determined using a Dohrmann DC-180 carbon analyzer. Ultraviolet absorption at 254 nm (UVA) was measured using a Perkin Elmer Lambda 2 UV/VIS scanning spectrophotometer.
| qe = x/m = KCe1/n | (1) |
| log (x/m) = log K + 1/n log Ce | (2) |
| qe = K(Ce/Do)1/n | (3) |
The quantity of contaminant adsorbed and the equilibrium contaminant concentration were expressed in terms of fractional reduction in ultraviolet absorption or fluorescence relative to the initial (control) solution. Ultraviolet or fluorescence responses were surrogate parameters assumed to be proportional to concentration. Therefore, qe, the carbon capacity, was expressed as the fractional spectroscopic response adsorbed per mg of carbon added. In the traditional Freundlich model, Ce was represented by the fractional spectroscopic response remaining in solution, while the expression Ce/Do in the modified Freundlich model was calculated by dividing the fractional spectroscopic response remaining in solution by the adsorbent dose.
Individual isotherms were calculated by linear regression for each duplicate analyzed at 24 and 48 h within each type of humic substance and subfraction investigated. In order to determine if the data from individual isotherms could be pooled, the concept of introducing dummy variables22–24 into a multiple regression model of eqn. 2 (and analogously for the modified Freundlich model) was applied using the Statistical Analysis System (SAS). Sets of 4 isotherms were compared simultaneously using
| log (x/m) = log K + Z1 + Z2 + Z3 + 1/n log Ce + Z1 log Ce + Z2 log Ce + Z3 log Ce | (4) |
F-400 activated carbon (60 × 80), supported by silane treated glass wool and teflon boiling chips, was added to a teflon column having a 1.12 cm inner diameter. A double head, positive displacement pump (Cole-Parmer, Chicago, IL) fed the humic solution to the column from the 200 L reservoir. Columns having EBCTs of 1.02 and 2.05 min were run simultaneously. To alleviate from air bubbles potentially being introduced into the column, a gas trap was prepared and fed by a third pump head placed on the same shaft as the column influent pump heads. The tubing size of the third pump head was larger than that of the column influent heads, so the flow into the gas trap was greater than the flow out of the gas trap. The effluent of the gas trap was connected to the pump heads feeding the minicolumns. A side arm flask was used as the gas trap so the overflow from the flask could be recycled to the influent reservoir.
Flow Field-Flow Fractionation Analysis
The flow FFF apparatus used in this laboratory10,11 was modified for this research to include automated injection, automated flow switching, and fluorescence detection (Fig. 1). A 2 position, 6 port, double, 3 way valve (Rheodyne), pneumatically actuated as a timed event, was installed to automate switching the solvent flow path that previously10,11 was switched manually (Fig. 2). The autosampler of a Hewlett-Packard Model 1090M liquid chromatograph was used to inject samples. The software provided with the liquid chromatograph was used to program a method sequence that included automated sample injection and control of the Rheodyne valve for flow switching. The autosampler introduced a substantial amount of back-pressure into the system that was offset through the use of pressure restrictors, as shown in Fig. 1, to maintain balanced pressures throughout the flow FFF system. | ||
| Fig. 1 Schematic of automated flow FFF system. | ||
 | ||
| Fig. 2 Schematic of double three-way valve. | ||
The cross flow was used as the field of separation. The sample was injected into the system and carried into the fractionator by the channel flow. Parallel to this flow a semipermeable membrane was located along one side of the channel. After the sample was injected, the channel flow was diverted, allowing a cross flow, introduced opposite to the membrane, to drive the macromolecular species to one side of the channel. The cross flow fluid exited the channel through the membrane while the macromolecules accumulated along the membrane. Following the stop flow period, the longitudinal (or channel) flow was reintroduced into the channel.
High-performance liquid chromatographic pumps and detectors (Hewlett-Packard) were utilized with a flow FFF channel, Model F-1000, manufactured by FFFractionation (Salt Lake City, UT). The flow FFF channel dimensions were a tip-to-tip length of 28.5 cm, a width of 2.0 cm, and a thickness of 0.0508 cm. The membrane consisted of polypropylene backed polysulfone, type PM10F, manufactured by Amicon (Beverly, MA), having a molecular weight cutoff of 10,000. Flow FFF was conducted with an injection volume of 250 µL, a cross flow rate of 0.4 mL min−1, a channel flow rate of 0.2 mL min−1, and a stop flow period of 7.5 min. The sample was completely contained within the channel after 2.5 min; thus, the stop flow was initiated after 2.5 min and terminated after 10 min. The mobile phase consisted of 0.095 M dibasic potassium phosphate, 0.005 M monobasic potassium phosphate, and 0.03 percent sodium azide in HPLC grade water (pH 8.1). Diode array detection of each sample was performed at 220, 254, 270, 300, 330, 370, and 400 nm. The fluorescence of all samples was measured at excitation and emission wavelengths of 248 and 443 nm, respectively. Under these conditions, a detection limit of 0.5 mg L−1 was established for both diode array and fluorescence detection of DOC.
Results and discussion
In order to characterize the complexities of DOM, researchers utilize appropriate analytical tools to fractionate these heterogeneous substances into physically and chemically meaningful molecular subsets and to detect the resultant pieces of the puzzle. Acid-base chemistry is used to separate fulvic from humic acids, and pyrolysis gas chromatography generates identifiable molecular fragments of DOM. To separate DOM based on hydrophobicity and/or ionogenicity, interaction with sorbents such as activated carbon, ion exchange, or hydrophobic sorbents such as XAD resins, reversed-phase bonded silica sorbents for solid phase extraction and high-performance liquid chromatography, and supercritical fluid extractants, are used. Capillary electrophoresis is used to separate on the basis of charge migration; size exclusion chromatography is used to fractionate DOM by molecular size; and flow FFF is used to separate macromolecules from non-macromolecules and to separate macromolecules on the basis of diffusivity. Once fractionated, DOM is detected by ultraviolet (UV) absorption, fluorescence, Raman spectroscopy, nuclear magnetic resonance spectroscopy, and mass spectrometry.This research fractionated commercially available humic substances into DOM subfractions based on filtration at 0.22 µm, adsorptivity to activated carbon, and molecular size/diffusivity/charge by flow FFF. Humic and fulvic acids were separated by acid-base chemistry (Fig. 3, Path I). The DOM samples that did not contact activated carbon (isotherm controls) were analyzed at 254 nm (UVA) and by flow FFF (Fig. 3, Path II), as were the solutions remaining in equilibrium after contact with activated carbon (Fig. 3, Path III). Selective detectors were used to differentiate between types of molecules.
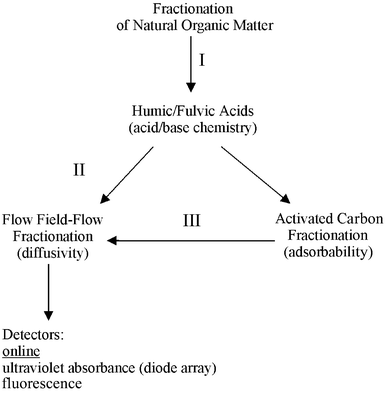 | ||
| Fig. 3 Techniques used in this research for the fractionation of organic matter. | ||
Prior to fractionation by flow FFF, DOM solutions are assumed to consist of 4 broad categories of molecules based on molecular size and spectroscopic properties: (1) UV absorbing, non-fluorescing non-macromolecules; (2) UV absorbing, fluorescing non-macromolecules; (3) UV absorbing, non-fluorescing, polyelectrolytic macromolecules; and (4) UV absorbing, fluorescing polyelectrolytic macromolecules. The flow FFF retains only UV absorbing, polyelectrolytic macromolecules (both fluorescing and non-fluorescing) from categories 3 and 4 that are sensed by the diode array detector (FFF-DAD). The fluorescence detector (FFF-FLD), situated in tandem sequence after the FFF-DAD, detects only UV absorbing, fluorescing, polyelectrolytic macromolecules, category 4.
The raw output from flow FFF is referred to as a “fractogram,” analogous to the term “chromatogram,” used for liquid or gas chromatography. The FFF-DAD fractogram is a plot of UV absorbance versus time, and the FFF-FLD fractogram is a plot of fluorescence versus time. FFF-DAD and FFF-FLD fractograms were obtained by simultaneously monitoring a single sample injection because the DAD and FLD were connected in tandem. Fractograms presented in this publication consist of raw data output that is not electronically retouched.
Even though the molecular weight cutoff is 10,000 for the semipermeable membrane used for flow FFF, the negatively charged polysulfone membrane repels negatively charged polyelectrolytic humic macromolecules causing them to be retained in the channel. Previously it was demonstrated that fulvic and humic acids ranging in weight-average molecular weight from 750 to 3400 were retained in this system.10
Adsorbate solutions were prepared by adding an initial mass of 20 mg of organic matter to 1 L of buffer solution. However, due to solubility differences in individual humic substances and subsequent to filtration to a particle size less than 0.22 µm, the average initial DOC concentrations were 5.100 mg L−1 for Aldrich humic acid at pH 6; 8.197 mg L−1 for Aldrich humic acid at pH 9; 8.787 mg L−1 for Aldrich humic extract at pH 6; and 10.628 mg L−1 for Suwannee fulvic acid at pH 6. DOM levels are expected to be approximately double the amount of DOC.25 Aldrich humic acid was more soluble at pH 9 than pH 6 presumably because more functional groups are ionized and become more water soluble at the higher pH. The Aldrich humic extract (fulvic components) and Suwannee River fulvic acid were more soluble relative to the humic acids due to lower molecular weight and reduced hydrophobicity.
Batch equilibrium (Adsorption Isotherm) studies
UVA measured at 254 nm is a single data point measuring the intensity of absorption at a specific wavelength in the spectrum of the humic substance in solution and is expected to be proportional to DOC concentration. FFF-DAD-254 represents the area under the curve of the flow FFF fractogram (analogous to a chromatogram) measured at 254 nm and is also expected to be proportional to DOC concentration. Multiple wavelengths were monitored by FFF-DAD, but only the results at 254 nm (FFF-254) were used to generate isotherms in comparison to UVA.
There is, however, a fundamental chemical difference in the humic substances measured by UVA and FFF-DAD-254. UVA measured prior to further fractionation monitors UV absorbing, fluorescing and non-fluorescing, macromolecules and non-macromolecules (categories 1–4). FFF-DAD-254 represents only fluorescing and non-fluorescing macromolecules (categories 3 and 4) because low molecular weight, non-polyelectrolytic, non-macromolecules are not retained within the FFF channel. Thus, they would each be proportional to a different DOC concentrations. To support the conclusion that FFF-DAD-254 represents a subset of molecules relative to measured UVA data, plots of these two parameters for isotherm control data only (Fig. 4) and for all isotherm data (Fig. 5) are examined.
 | ||
| Fig. 4 Comparison of molecular subsets of humic substances (a) Aldrich humic acid extract (pH 6), (b) Aldrich humic acid (pH 9) and (c) Suwannee River fulvic acid (pH 6) from control isotherm solutions. | ||
 | ||
| Fig. 5 Comparison of UVA and FFF-DAD-254 measurements of the equilibrium concentrations remaining in solution following batch isotherm tests for humic substances (a) Aldrich humic extract (pH 6), (b) Aldrich humic acid (pH 9), (c) Aldrich humic acid (pH 6) and (d) Suwannee River fulvic acid (pH 6). The correlation coefficient (r2) for each plot represents the quadratic expression. | ||
Comparison of UV absorbing characteristics by UVA and FFF-DAD-254, with each parameter normalized to the DOC concentration, reveals that the data do not show a linear trend intersecting the origin (Fig. 4). (SUVA is the UVA value divided by the DOC concentration. SUVA normalizes the UVA data to carbon and represents the amount of aromaticity per milligram of DOC.26) The SUVA value was weighted by a factor of 10,000 to adjust both parameters into the same order of magnitude. A hypothetical reference line with a slope of 1 was added to the fig. The relationship is not necessarily expected to have a slope of one, but SUVA and the normalized FFF-DAD-254 should be proportional to each other and intersect the origin if both parameters measure the same molecules. However, they do not in this graph (Fig. 4). This indicates that the DOC measured before flow FFF is not an accurate measure of the DOC retained in the channel. Therefore, the conclusion that different categories of molecules are measured by UVA and FFF-DAD-254 as assumed is supported.
Both SUVA and the normalized FFF-DAD-254/DOC show an increase in magnitude in the order Suwannee fulvic acid < Aldrich humic acid (pH 9) < Aldrich humic acid extract (pH 6). Since the relationship in Fig. 4 tends toward intersecting the x-axis at a positive value of SUVA where FFF-DAD-254/DOC is zero, the x-intercept value should represent the non-macromolecular organic matter that is not observed in flow FFF. This supports the original assumption that the solutions of DOM in these products as received contain all four categories of molecular types. Fig. 4 may also be interpreted to mean that the farther away a point is (in the y-direction) from the hypothetical line of slope 1 intersecting the origin, the smaller the ratio of macromolecules/non-macromolecules is. An alternative explanation of results that cannot be discounted is that the UV extinction coefficients of the macromolecules may differ in each humic substance type. All of these effects could be operative simultaneously. For example, although the pre-FFF DOC of the Suwannee fulvic acid was 11.3 mg L−1, the FFF-DAD-254 peak was smaller than that observed for the Aldrich humic acid, which had a pre-FFF DOC of 5.9 mg L−1. This probably represents a combination of differing molecular subsets as well as UV extinction coefficient differences.
Using the data from all isotherm experiments, UVA measurements are compared to the integrated fractogram areas at 254 nm (Fig. 5). The data measure the equilibrium concentrations of humic substances remaining in solution following batch isotherm tests. The assumption was made earlier that UVA data represent all four categories of molecular types potentially present, while FFF-DAD-254 measurements represent only macromolecules from categories 3 and 4. The curvilinear nature of the relationship between data presented in Fig. 5 indicates that the UVA data and the FFF-DAD-254 data do not represent the same molecular populations as assumed. In each case, when quadratic expressions were applied, the relationship was clearly nonlinear with correlation coefficients (r2) greater than 0.9 for each humic substance. Also, it is important to note in Fig. 5 that a different relationship exists for each different humic substance type, clearly indicating that this two-dimensional comparison is capable of discriminating among humic substances of different molecular character.
 | ||
| Fig. 6 Fractogram overlays of Aldrich humic acid isotherms on activated carbon at pH 6 by (a) ultraviolet absorption (270 nm) or (b) fluorescence detection or at pH 9 by (c) ultraviolet absorption (270 nm) or (d) fluorescence detection. In the fractogram, A represents 64 mg of activated carbon added, B-32 mg, C-16 mg, D-4 mg, and E-0 mg. | ||
When the DAD and FLD fractograms of Aldrich humic acid adsorbed on activated carbon are compared at a given pH (compare Fig. 6a to 6b and compare Fig. 6c to 6d), the preferential adsorption of fluorescing macromolecules is apparent. Similar results were obtained12 for the Aldrich humic acid extract (pH 6) and Suwannee River fulvic acid (pH 6), using isotherm fractogram overlays and comparison of DAD/FLD area ratios to activated carbon dose (data not presented). Therefore, the fluorescing components of fulvic or humic acids are preferentially adsorbed.
The effect of the pH at which the isotherm was conducted is apparent by comparing the FFF-DAD-270 fractograms, Fig. 6a with 6c, and, analogously, by comparing the FFF-FLD fractograms, Fig. 6b with 6d. The magnitude of both UV absorbance (Fig. 6avs. 6c) and fluorescence (Fig. 6bvs. 6d) was decreased to a greater extent per unit mass of activated carbon at pH 6 than at pH 9.
 | ||
| Fig. 7 Adsorption isotherm equilibrium concentrations of humic substances (a) Aldrich humic acid (pH 6), (b) Aldrich humic acid (pH 9), (c) Aldrich humic acid extract (pH 6) and (d) Suwannee River fulvic acid (pH 6). Fractional reduction of UVA is represented by open diamonds; of FFF-DAD-254 by closed triangles; and of FFF-FLD by closed squares. | ||
At 16 mg of activated carbon added, the fractional fluorescence response was at or below 0.1 for all substances measured at pH 6 (for Aldrich humic acid, Aldrich humic acid extract, and Suwannee River fulvic acid). However, the fractional response for Aldrich humic acid at pH 9 was 0.2, indicating that at the higher pH the fluorescent components were less adsorbed to the activated carbon.
Using the UVA data, the independent x variable in the equation y = mx + b (the equilibrium concentration in the Freundlich model or the dose normalized equilibrium concentration in the modified Freundlich model), was not significant at α = 0.05 in 3 of 16 comparisons for the Freundlich model or in 1 of 16 comparisons for the modified Freundlich model. Likewise, the x variable was not significant in 14 of 16 comparisons for the FFF-DAD-254 isotherms for the Freundlich model or in 9 of 16 comparisons for the modified Freundlich model. Finally, the x variable was not significant for FFF-FLD data in 5 of 16 comparisons for the Freundlich model or in 3 of 16 comparisons for the modified Freundlich model. Therefore, it was concluded that the Freundlich and the modified Freundlich models were appropriate for describing the UVA and the FFF-FLD-derived data. However, the FFF-DAD-254 isotherms did not adequately describe the data observed. The results are considered complementary: it seems reasonable to assume that the FFF-DAD-254 data are not modeled by the Freundlich equations because FFF-FLD data represent a molecular subset of the FFF-DAD-254 data, and the FFF-FLD data exhibit preferential adsorption. Thus, only the isotherms generated by UVA data (representing fluorescing and non-fluorescing macromolecules and non-macromolecules) and those generated using FFF-FLD data (representing fluorescing macromolecules) are reported (Table 1). To allow for direct comparison of the two methods, the traditional Freundlich model parameters are not italicized in Table 1 while the modified Freundlich model parameters are. The use of the modified Freundlich model improved the correlation coefficients (r2) relative to those determined by the traditional Freundlich model. Generally, however, the fundamental conclusions drawn in this report were the same regardless of the model used.
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (FFF-FLD) Fluorescing macromolecules | Individual | Pooled | ||||||||
| Test | Day | # | 1/n | K | r 2 | # | 1/n | K | r 2 | |
| a Freundlich model parameters (eqn. 1) are listed in regular font; modified Freundlich model parameters (eqn. 3) are listed in italic font. | ||||||||||
| Suwannee River fulvic acid (pH 6.0) | 1 | 1 | 3 | 0.273 | 0.115 | 0.9859 | ||||
| 0.190 | 0.152 | 0.9990 | ||||||||
| 1 | 2 | 3 | 0.289 | 0.118 | 0.9964 | |||||
| 0.196 | 0.154 | 0.9997 | ||||||||
| 2 | 1 | 3 | 0.252 | 0.115 | 0.9671 | |||||
| 0.182 | 0.151 | 0.9927 | ||||||||
| 2 | 2 | 3 | 0.254 | 0.0960 | 0.9792 | |||||
| 0.174 | 0.125 | 0.9988 | ||||||||
| 12 | 0.262 | 0.109 | 0.9578 | |||||||
| 12 | 0.184 | 0.143 | 0.9811 | |||||||
| Aldrich humic acid (pH 6.0) | 1 | 1 | 2 | 0.315 | 0.272 | – | ||||
| 0.224 | 0.330 | – | ||||||||
| 1 | 2 | 3 | 0.464 | 0.375 | 0.9192 | |||||
| 0.305 | 0.462 | 0.9589 | ||||||||
| 2 | 1 | 2 | 0.346 | 0.301 | – | |||||
| 0.242 | 0.366 | – | ||||||||
| 2 | 2 | 2 | 0.251 | 0.181 | – | |||||
| 0.180 | 0.221 | – | ||||||||
| 9 | 0.352 | 0.271 | 0.8852 | |||||||
| 9 | 0.247 | 0.341 | 0.9283 | |||||||
| Aldrich humic extract (pH 6.0) | 1 | 1 | 3 | 0.442 | 0.232 | 0.9527 | ||||
| 0.279 | 0.296 | 0.9692 | ||||||||
| 1 | 2 | 4 | 0.495 | 0.231 | 0.9994 | |||||
| 0.307 | 0.307 | 0.9980 | ||||||||
| 2 | 1 | 4 | 0.513 | 0.229 | 0.9950 | |||||
| 0.317 | 0.313 | 0.9989 | ||||||||
| 2 | 2 | 4 | 0.406 | 0.177 | 0.9621 | |||||
| 0.269 | 0.244 | 0.9758 | ||||||||
| 15 | 0.466 | 0.215 | 0.9640 | |||||||
| 15 | 0.296 | 0.292 | 0.9795 | |||||||
| Aldrich humic acid (pH 9.0) | 1 | 1 | 4 | 1.13 | 0.310 | 0.9653 | ||||
| 0.462 | 0.361 | 0.9822 | ||||||||
| 1 | 2 | 4 | 0.976 | 0.214 | 0.9977 | |||||
| 0.421 | 0.280 | 0.9948 | ||||||||
| 2 | 1 | 4 | 1.03 | 0.236 | 0.9629 | |||||
| 0.438 | 0.304 | 0.9790 | ||||||||
| 2 | 2 | 4 | 1.04 | 0.206 | 0.9991 | |||||
| 0.433 | 0.275 | 0.9973 | ||||||||
| 16 | 1.03 | 0.232 | 0.9652 | |||||||
| 0.437 | 0.301 | 0.9831 | ||||||||
| (b) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (UVA) Fluorescing and non-fluorescing macromolecules and mon-macromolecules | Individual | Pooled | ||||||||
| Test | Day | # | 1/n | K | r 2 | # | 1/n | K | r 2 | |
| Suwannee River fulvic acid (pH 6.0) | 1 | 1 | 4 | 0.274 | 0.0780 | 0.9999 | ||||
| 0.188 | 0.107 | 0.9919 | ||||||||
| 1 | 2 | 4 | 0.271 | 0.0738 | 0.9918 | |||||
| 0.186 | 0.102 | 0.9946 | ||||||||
| 2 | 1 | 4 | 0.226 | 0.0620 | 0.9913 | |||||
| 0.158 | 0.0825 | 0.9878 | ||||||||
| 2 | 2 | 4 | 0.432 | 0.0805 | 0.8591 | |||||
| 0.248 | 0.117 | 0.9140 | ||||||||
| 16 | 0.264 | 0.0683 | 0.8826 | |||||||
| 16 | 0.184 | 0.0964 | 0.9292 | |||||||
| Aldrich humic acid (pH 6.0) | 1 | 1 | 4 | 0.432 | 0.0977 | 0.9458 | ||||
| 0.262 | 0.144 | 0.9800 | ||||||||
| 1 | 2 | 4 | 0.305 | 0.0977 | 0.9651 | |||||
| 0.210 | 0.136 | 0.9869 | ||||||||
| 8 | 0.327 | 0.0898 | 0.8719 | |||||||
| 0.224 | 0.133 | 0.9430 | ||||||||
| 2 | 1 | 4 | 0.302 | 0.0526 | 0.8824 | |||||
| 0.198 | 0.0784 | 0.9762 | ||||||||
| 2 | 2 | 4 | 0.320 | 0.0611 | 0.9930 | |||||
| 0.197 | 0.0854 | 0.9926 | ||||||||
| 8 | 0.308 | 0.0563 | 0.9229 | |||||||
| 0.197 | 0.0815 | 0.9757 | ||||||||
| Aldrich humic extract (pH 6.0) | 1 | 1 | 4 | 0.530 | 0.0473 | 0.9352 | ||||
| 0.250 | 0.0723 | 0.9981 | ||||||||
| 1 | 2 | 4 | 0.239 | 0.0351 | 0.9394 | |||||
| 0.145 | 0.0470 | 0.9974 | ||||||||
| 2 | 1 | 4 | 0.279 | 0.0342 | 0.9608 | |||||
| 0.144 | 0.0440 | 0.9062 | ||||||||
| 2 | 2 | 4 | 0.167 | 0.0429 | 0.6625 | |||||
| 0.139 | 0.0611 | 0.8198 | ||||||||
| 16 | 0.192 | 0.0348 | 0.5672 | |||||||
| 16 | 0.146 | 0.0500 | 0.7590 | |||||||
| Aldrich humic acid (pH 9.0) | 1 | 1 | 4 | 0.394 | 0.0497 | 0.9643 | ||||
| 0.213 | 0.0717 | 0.9938 | ||||||||
| 1 | 2 | 4 | 0.512 | 0.0769 | 0.9455 | |||||
| 0.280 | 0.120 | 0.9964 | ||||||||
| 2 | 1 | 4 | 0.448 | 0.0506 | 0.9124 | |||||
| 0.242 | 0.0790 | 0.9915 | ||||||||
| 12 | 0.224 | 0.0754 | 0.9886 | |||||||
| 2 | 2 | 4 | 0.413 | 0.0526 | 0.9785 | |||||
| 0.221 | 0.0765 | 0.9969 | ||||||||
| 16 | 0.431 | 0.0554 | 0.8845 | |||||||
Individual isotherms were compared using the “dummy variable” approach described in the Experimental section. In instances in which it was statistically determined that the slopes and intercepts represented the same populations, the data were pooled (Table 1). For each instance of pooled data reported in Table 1, the dependent variable was significant at α = 0.05.
The isotherm data showed no difference between the experiments conducted for 1 day or 2 days. In only one instance (UVA data for Aldrich humic acid (pH 6.0)), the analysis demonstrated a difference between the replications of test 1 and test 2.
If the traditional or modified Freundlich models adequately represent the data, the plot should be linear, and the parameters 1/n and K can be obtained from the slope and intercept, respectively (eqn. 2). The adsorption capacity of activated carbon for organic compounds is found to be larger with increasing values of K while the intensity of adsorption (adsorbability) is higher as n increases (or as 1/n decreases).18 This means that with higher adsorption capacity the isotherm is shifted upward, and with a stronger adsorption bond, the slope of the isotherm becomes flatter (Fig. 8). In these results, the maximum adsorption capacity, or K value, can be interpreted to represent the fractional absorbance (or fluorescence) adsorbed per mg of activated carbon from 100 mL solutions of adsorbate.
 | ||
| Fig. 8 Modified Freundlich model isotherm parameters (pooled) for fluorescing macromolecules. The y-axis represents log K (adsorption capacity) and the slope is 1/n (adsorbability) after eqn. 2: (a) Aldrich humic acid (pH 6), (b) Suwannee River fulvic acid (pH 6), (c) Aldrich humic acid extract (pH 6), and (d) Aldrich humic acid (pH 9). | ||
For fluorescing macromolecules (Table 1a), the fractional adsorption capacity (pooled, modified Freundlich) ranged from a low K value of 0.143 for Suwannee River fulvic acid (pH 6) to a high K value of 0.341 for Aldrich humic acid at pH 6, indicating that the adsorption capacity of activated carbon was least for Suwannee River fulvic acid at pH 6 (Fig. 8). In fact, the capacity for fluorescing macromolecules from Aldrich humic acid (pH 6) was more than double the capacity for Suwannee River fulvic acid. Conversely, the adsorbability of fluorescing macromolecules was greatest for the Suwannee River fulvic acid at pH 6 (1/n = 0.184) and least for Aldrich humic acid at pH 9 (1/n = 0.437). The adsorption capacity of activated carbon toward Aldrich humic acid was greater at pH 6 than at pH 9 (K = 0.341 at pH 6 and K = 0.301 at pH 9), while the adsorbability was markedly greater at pH 6 than at pH 9 (1/n = 0.247 at pH 6 and 1/n = 0.437 at pH 9).
For UVA data that includes all fluorescing and nonfluorescing macromolecules and nonmacromolecules (Table 1b), the fractional adsorption capacity (pooled, modified Freundlich) demonstrated little variability, ranging from K values of 0.05 to 0.133 with only one value determined to be greater than 0.1. Fulvic components, the Aldrich humic extract at pH 6 (1/n = 0.146) and the Suwanee fulvic acid at pH 6 (1/n = 0.184) demonstrated greater adsorbability than humic components.
Table 1a presents data unique in the literature because activated carbon isotherm parameters isolated for fluorescing macromolecular components of DOM have not previously been reported. However, Table 1b, based on UVA data measured for activated carbon adsorption of combined fluorescing and non-fluorescing macromolecules and non-macromolecules corresponds to isotherm data more commonly reported in the literature.
Minicolumn breakthrough studies
As expected, in the activated carbon minicolumn experiments, UVA breakthrough occurred more rapidly with a lower EBCT. The humic acid was detected in the outlet stream at approximately 4 h for an EBCT of 1.02 min and at about 9 h for an EBCT of 2.05 min. Moreover, the curve rose much more sharply for an EBCT of 1.02 min.Both columns tested appeared to reach steady state at approximately 45 h when the concentration no longer changed appreciably with time. The effluent concentration never reached the influent concentration, suggesting that a fraction of the humic material was irreversibly adsorbed, i.e., a biphasic adsorption process occurred.
Fluorescing species (FFF-FLD) demonstrated slower breakthrough relative to UV absorbing, nonfluorescing macromolecules (Fig. 9). The minicolumn influent has the greatest concentration of organic matter detected by FFF-DAD (Fig. 9a) or by (FFF-FLD, Fig. 9b). The other peaks in the overlay of Fig. 9 represent the breakthrough measurements of the minicolumn effluent. As breakthrough from the minicolumn occurs, the area under the peak of the fractogram increases. In only one fraction collected from the minicolumn study is appreciable fluorescence detected in Fig. 9b relative to the UV absorption in Fig. 9a. Because the DAD and FLD detectors are arranged in tandem, the data in Fig. 9a and 9b visualize the same samples injected. As observed in the batch equilibrium studies, the minicolumn experiments also indicate preferential adsorption of fluorescing macromolecules.
 | ||
| Fig. 9 Fractogram overlays of Aldrich humic acid (pH 6) minicolumn adsorption breakthrough studies on activated carbon (EBCT 2.046 min) by (a) FFF-DAD-270 or (b) FLD. | ||
Conclusions
The preferential adsorption on activated carbon of fluorescing macromolecular components of both humic and fulvic acids was demonstrated using batch equilibrium adsorption studies and minicolumn adsorption breakthrough studies. Activated carbon adsorption exhibited less capacity yet greater adsorbability toward fulvic fluorescing macromolecules relative to humic fluorescing macromolecules when modeled with modified Freundlich model adsorption isotherms. Comparing the graphitic molecular character of activated carbon and the molecular characteristics that impart fluorescence properties to organic compounds, it seems reasonable to conclude that the preferential adsorption of fluorescing macromolecules on activated carbon is scientifically based in adsorption processes.References
- A. Piccolo, in The Role of Humic Substances in the Ecosystems and in Environmental Protection, ed. J. Drozd, S. S. Gonet, N. Senesi and J. Weber, IHSS-Polish Society of Humic Substances, Wroclaw, Poland, 1997, pp. 19–35 Search PubMed.
- E. Tombacz, J. A. Rice and S. Ren, in The Role of Humic Substances in the Ecosystems and in Environmental Protection, ed. J. Drozd, S. S. Gonet, N. Senesi and J. Weber, IHSS-Polish Society of Humic Substances, Wroclaw, Poland, 1997, pp. 43–50 Search PubMed.
- D. M. Owen, G. L. Amy and Z. K. Chowdhury, Characterization of Natural Organic Matter and Its Relationship to Treatability, American Water Works Association Research Foundation, Denver, Colorado, 1993 Search PubMed.
- R. L. Malcolm, Anal. Chim. Acta, 1990, 232, 19 CrossRef CAS.
- J. C. Crittenden, P. S. Reddy, D. W. Hand and H. Arora, Prediction of GAC Performance Using Rapid Small-Scale Column Tests, American Water Works Association Research Foundation, Denver, Colorado, 1989 Search PubMed.
- R. S. Summers, L. Cummings, J. DeMarco, D. J. Hartman, D. H. Metz, E. W. Howe, B. MacLeod and M. Simpson, Standardized Protocol for the Evaluation of GAC, American Water Works Association Research Foundation, Denver, Colorado, 1992 Search PubMed.
- R. Beckett, Environ. Technol. Lett., 1987, 8, 339 Search PubMed.
- R. Beckett, Z. Jue and J. C. Giddings, Environ. Sci. Technol., 1987, 21, 289 CAS.
- R. Beckett, in Aquatic Humic Substances Influence on Fate and Treatment of Pollutants. Advances in Chemistry Series No. 219, ed. I. H. Suffet and P. MacCarthy, American Chemical Society, Washington, DC, 1989, pp. 65–80 Search PubMed.
- P. J. M. Dycus, K. D. Healy, G. K. Stearman and M. J. M. Wells, Sep. Sci. Technol., 1995, 30(7–9), 1435 Search PubMed.
- M. J. M. Wells, K. D. Healy, G. K. Stearman and P. J. M. Dycus, in Advances in Filtration and Separation Technology, ed. K.-J. Choi, The American Filtration and Separations Society, Northport, AL, 1995, vol. 9, pp. 438–450 Search PubMed.
- K. D. Healy, Master's Thesis, Tennessee Technological University, 1995.
- M. C. Lee, V. L. Snoeyink and J. C. Crittenden, J. Am. Water Works Assoc., 1981, 73(8), 440 Search PubMed.
- J. E. Kilduff, T. Karanfil, Y.-P. Chin and W. J. Weber, Jr., Environ. Sci. Technol., 1996, 30, 1336 CrossRef CAS.
- K. Kaiser and W. Zech, in The Role of Humic Substances in the Ecosystems and in Environmental Protection, ed. J. Drozd, S. S. Gonet, N. Senesi and J. Weber, IHSS-Polish Society of Humic Substances, Wroclaw, Poland, 1997, pp. 385–390 Search PubMed.
- A. J. Stewart and R. G. Wetzel, Limnol. Oceanogr., 1980, 25, 559 Search PubMed.
- N. Plechanov, Org. Geochem., 1983, 5(3), 143 CrossRef CAS.
- V. L. Snoeyink, in Water Quality and Treatment, ed. F. W. Pontius, McGraw-Hill, Inc., New York, 4th edn., 1990 Search PubMed.
- W. Stumm, Chemistry of the Solid-Water Interface, John Wiley & Sons, Inc., New York, 1992, pp. 87–155 Search PubMed.
- J. E. Kilduff, T. Karanfil and W. J. Weber, Jr., Environ. Sci. Technol., 1996, 30, 1344 CrossRef CAS.
- T. Karanfil, M. Kitis, J. E. Kilduff and A. Wigton, Environ. Sci. Technol., 1999, 33, 3225 CrossRef CAS.
- N. R. Draper and H. Smith, Applied Regression Analysis, John Wiley & Sons, Inc., New York, 1967, pp. 134–141 Search PubMed.
- J. H. Zar, Biostatistical Analysis, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1974, p. 268 Search PubMed.
- R. G. D. Steel and J. H. Torrie, Principles and Procedures of Statistics: A Biometrical Approach, 2nd edn. McGraw-Hill, New York, 1980, pp. 298, 407 and 444 Search PubMed.
- R. P. Schwarzenbach, P. M. Gschwend and D. M. Imboden, Environmental Organic Chemistry, John Wiley & Sons, Inc., New York, 1993, pp. 266, 268 Search PubMed.
- R. Fujii, A. J. Ranalli, G. R. Aiken and B. A. Bergamaschi, US Geological Survey Water-Resources Investigations Report 98-4147, 1998. Available online: http://water.wr.usgs.gov/rep/wrir984147/.
| This journal is © The Royal Society of Chemistry 2002 |
