Total organic carbon analysis as a precursor to disinfection byproducts in potable water: Oxidation technique considerations
Brian
Wallace
,
Mike
Purcell
and
Joe
Furlong
Tekmar-Dohrmann, 4736 Socialville-Fosters Road, Mason, Ohio 45040, USA. E-mail: Brian.Wallace@EmersonProcess.com; Tel: +1 513 229 7068; Fax: +1 513 229 7050; Mike.Purcell@EmersonProcess.com; Tel: +1 513 229 8240Joe.Furlong@EmersonProcess.com; Tel: +1 513 229 8253
First published on 20th December 2001
Abstract
In recent years, an increasing number of regulations and methodologies have begun to utilize total organic carbon (TOC) analysis for monitoring microbial contamination and/or disinfectant byproduct (DBP) precursors. This paper highlights some analytical differences and similarities between the two widely used TOC oxidation techniques: UV persulfate and high temperature combustion (HTC). Previous papers have come to different and sometimes contradictory conclusions on this subject. However, these studies either compared instruments with significantly different flow paths or TOC systems from different eras. Unlike previous studies, this paper compares two modern TOC analyzers with nearly identical flow paths for sample recovery, detection limits, and analysis of real world samples. On average, both persulfate and HTC oxidation yielded good recoveries for 10 hard to oxidize compounds and potable water samples from 5 different locations across the USA. In general, persulfate yielded more precise results because of its lower background response relative to sample response while HTC gave slightly higher results (roughly 2% to 3%) for surface water samples.
 Brian Wallace Brian Wallace |
 Mike Purcell Mike Purcell |
 Joe Furlong Joe Furlong |
1 Introduction
In drinking water treatment facilities, the process of treating source water (e.g., lake, river, reservoir, or groundwater) to kill pathogens is known as disinfection. Typically, chlorine, hypochlorite, ozone, chlorine dioxide, and chloramines are the chemicals used for disinfection. These disinfectants perform an effective job against pathogen microorganisms; however, these same protective disinfectants can create, in certain conditions, byproducts that are potentially carcinogenic or pose other harmful health effects. Some of these effects have been studied in animal toxicology.1 Disinfection byproducts, such as trihalomethanes and haloacetic acids, are known to be formed when certain disinfectants react with natural organic matter (NOM) in source water.In April 2000, the US EPA published recent revisions of the Stage 1 Disinfectants/Disinfection Byproducts (D/DBP) Rule to reduce the public risk of exposure to DBPs. The Stage 1 D/DBP Rule calls for the monitoring and reduction of NOM, a precursor to DBPs. It is accepted that, by reducing the NOM before disinfection, the formation of DBPs will be reduced. For measuring NOM, total organic carbon (TOC) analysis was chosen. By December 2003, all conventional public water systems that use surface water as their source, are required to monitor TOC and, if necessary, practice enhanced coagulation and enhanced softening to lower the TOC level. TOC monitoring is required monthly for one source water and one treated water sample. The amount of TOC removal from source water is dependent on the alkalinity and TOC concentrations, as described in Table 1. If the TOC levels of the source water average less than 2 mg L−1 C for two consecutive years, or the treated water averages less than 1 mg L−1 C for 1 year, then a public water system would qualify for reduced monitoring to one pair of samples per quarter.2,3
With the introduction of the Stage 1 D/DBP Rule and the Interim Enhanced Surface Water Treatment Rule (IESWTR), the EPA has strengthened the importance of analyzing TOC as a precursor to D/DBPs. As shown in Table 1, the need for accurate TOC measurements for source and treated water is amplified by the Stage 1 D/DBP Rule. For example, an accurate measurement of TOC could potentially save a public water system from using higher dosages of coagulants because of an inaccurate higher measurement.
The Stage 1 D/DBP Rule calls for the use of Standard Method 5310 as the approved analytical method for TOC analysis.2 Two of the major oxidation technologies used today for TOC analysis are described in Standard Method 5310: high temperature combustion (HTC) and low temperature persulfate oxidation.4 The late 1980s saw the start of a major debate concerning which technique was best suited for testing TOC. This paper is designed to highlight the advantages and disadvantages of both techniques. The use of modern TOC instrumentation that contains the same flow paths but different oxidation techniques will be examined for hard to oxidize samples, detection limits, and real world samples.
2 Background: Methods and oxidation techniques
All laboratory TOC analyzers offered today use either the HTC method or persulfate oxidation. Both oxidation techniques either remove or measure the inorganic carbon (IC), defined as dissolved carbon dioxide, carbonate, and bicarbonate. One common technique measures non-purgeable organic carbon (NPOC), by introducing the sample and a small amount of inorganic acid to an inorganic carbon removal chamber. Through the acidification of the sample, the IC is converted to carbonic acid (H2CO3), also known as dissolved carbon dioxide (CO2). The sample is usually sparged (gas-stripped) with carrier gas to drive off any converted dissolved CO2. Loss of purgeable organic carbon (POC), in the form of volatile organic substances, can also occur during the sparging of the sample. Typically for many surface/drinking waters, the amount of POC is negligible and can be discounted.4 In these cases, NPOC is the equivalent of TOC. In cases where it is necessary to measure POC, the volatile organic molecules must be separated from the IC. After the sample is acidified and the IC converted to CO2, the CO2 is absorbed by a scrubber (typically containing soda lime). Subsequently, the POC continues to carry through the scrubber to an oxidation chamber. The POC is then oxidized to carbon dioxide and quantitated. For NPOC analysis, an oxidation step, following the removal of IC, converts the remaining non-volatile organic carbon in the solution to carbon dioxide. A detector measures the amount of carbon dioxide and applies that result to a calibration curve to attain the TOC value.The other method of measuring TOC requires the separate analysis for total carbon (TC) and IC. Known as TOC by difference, IC is subtracted from TC (TOC = TC−TIC). If present, POC will be included in the TC measurement. By the persulfate technique, TC is analyzed by adding acidified persulfate reagent to the sample for oxidation followed by sparging (gas-stripping) the carbon dioxide formed from the reaction. With the HTC technique, TC is analyzed by introducing sample into a combustion chamber at 680![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C or higher. For TC analysis of water samples containing carbonates, Standard Method 5310 states that, “Combustion temperatures above 950
°C or higher. For TC analysis of water samples containing carbonates, Standard Method 5310 states that, “Combustion temperatures above 950![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C are required to decompose some carbonates. Systems that use lower temperatures must destroy carbonates by acidification.”4
°C are required to decompose some carbonates. Systems that use lower temperatures must destroy carbonates by acidification.”4
For both oxidation techniques, IC is analyzed by the addition of inorganic acid to the sample. Phosphoric acid, or sulfuric acid as an alternative, are defined as the acids of choice as per the guidelines of Standard Method 5310.4 Following acidification, the sample is sparged to remove carbon dioxide created from acidification. The carbon dioxide formed in TC and IC analyses are both measured and applied to a calibration curve to attain the TC and/or IC value.
The NPOC method is the preferred way of analyzing TOC in drinking/surface waters. Table 2 highlights a mathematical comparison of the two methods used for the analysis of TOC. TOC by difference must perform two analyses per sample replicate compared with one replicate with NPOC analysis. Because of high IC contribution from samples, NPOC will yield more precise measurements compared to TOC by difference. As seen in Table 2, TOC by difference demonstrated a %RSD over four times as high as the precision value of NPOC on the same sample. NPOC is generally accepted as the equivalent of TOC because of the low POC content, which is usually less than 1% of the TOC value in drinking/surface water. TOC by NPOC analysis is also faster than TOC by difference. NPOC analysis time for most surface/drinking waters averages 4–6 min per replicate. Whereas, TOC by difference averages a 6–8 min analysis per replicate.
| Analysis type | Result/mg L−1 C | Avg. result/mg L−1 C | Standard deviation/mg L−1 C | %RSD | ||
|---|---|---|---|---|---|---|
| Rep. 1 | Rep. 2 | Rep. 3 | ||||
| TOC by difference (TC−IC)— | ||||||
| TC | 19.5000 | 19.1003 | 19.8998 | 19.8998 | 0.3998 | 2.05 |
| IC | 18.6711 | 18.3469 | 18.9953 | 19.0711 | 0.3242 | 1.70 |
| TOC = TC− IC | 0.8289 | 0.7534 | 0.9045 | 0.8289 | 0.0756 | 9.11 |
| TOC by NPOC— | ||||||
| TOC | 0.8286 | 0.7964 | 0.8125 | 0.8125 | 0.0161 | 1.98 |
Most official methods, for TOC analysis, describe their instrumentation requirements as seen in Table 3. All TOC analyzers today convert the organic carbon in the sample to carbon dioxide. The technique of detecting the resulting carbon dioxide varies and some detection techniques are not in all official methods.
| Oxidation | Detection technique | Analytical range/mg L−1 C | Official method |
|---|---|---|---|
| HTC | Thermal conductivity detector (TCD) | 5000–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
AOAC 955.07 |
| HTC | Coulometric | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–1 000–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
ASTM D4129 |
| HTC | Non-dispersive infrared (NDIR) | 0.004–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Standard Method 5310B; EPA 415.1, 9060A; ASTM D2579; ISO 8245; AOAC 973.47 |
| UV-persulfate | NDIR | 0.002–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Standard Method 5310C; EPA 415.2, 9060A; ASTM D2579; ISO 8245; AOAC 973.47 |
| Heated persulfate | NDIR | 0.002–1000 | Standard Method 5310C; EPA 415.2, 9060A; ASTM D2579; ISO 8245; AOAC 973.47 |
| UV-persulfate | Membrane/conductivity | 0.0005–50 | Standard Method 5310C |
The range of TOC measurements vary with oxidation method and detection technique. A HTC/thermal conductivity detector (TCD) method, used in many CHNS&O analyzers, may measure up to 1000 g L−1 C in a sample, whereas the non-dispersive infrared (NDIR) and conductivity detectors vary in range from as low as 0.5 µg L−1 to 25 g L−1 C.4 NDIR detectors measure the gaseous carbon dioxide formed from the oxidation of carbon. Some NDIR detectors have the advantage of sensitivity to low amounts of carbon, while not sacrificing the ability to analyze widely varying concentrations. The conductivity detectors are capable of measuring very low levels of carbon. However, they may require dilutions to measure many environmental samples and are sensitive to various interferences.
Halides and other ionic solutions are interferences that can damage both conductivity and NDIR detectors. Consequently, TOC analyzers that use NDIR detectors employ halide/corrosive scrubbers to prevent halide/corrosive inferences from reaching the detector. Conductivity detectors measure carbonic acid, which is the oxidation product of organic carbon in an aqueous state. Halide/corrosive scrubbers cannot be utilized with TOC analyzers that use conductivity detectors because these scrubbers would filter carbonic acid from the solution.
2.1 HTC oxidation technology
The HTC technique uses heat (680![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C or higher), usually in the presence of a catalyst, with a stream of hydrocarbon-free compressed air or oxygen to oxidize the organic carbon. Dissolved organics and particulate organics are expected to oxidize fully to carbon dioxide under these conditions. HTC instruments use a variety of different catalysts, including cupric oxide, cobalt oxide or titanium dioxide based platinum. HTC oxidation temperatures vary from 680 to 1000
°C or higher), usually in the presence of a catalyst, with a stream of hydrocarbon-free compressed air or oxygen to oxidize the organic carbon. Dissolved organics and particulate organics are expected to oxidize fully to carbon dioxide under these conditions. HTC instruments use a variety of different catalysts, including cupric oxide, cobalt oxide or titanium dioxide based platinum. HTC oxidation temperatures vary from 680 to 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C depending upon the application.
°C depending upon the application.
Once again, for TC analysis of surface/drinking water, combustion temperatures must be above 950![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for decomposition of some carbonates. NPOC analysis destroys carbonates by acidification and sparging.
°C for decomposition of some carbonates. NPOC analysis destroys carbonates by acidification and sparging.
2.2 Persulfate oxidation technology
Persulfate oxidation is a chemical oxidation aided by (i) UV radiation, (ii) UV radiation and persulfate, or (iii) heated persulfate. This characteristic of higher instrument response versus background response can yield a lower limit of detection versus the HTC technique.(a) Ultraviolet irradiation. In this technique, the sample is exposed to UV light from a mercury vapor lamp. All the dissolved organics may be oxidized to yield CO2. The maximum amount of TOC practically measured is 1 mg L−1 C with this technique.
(b) Heated persulfate. The sample is mixed with a quantity of persulfate solution and heated to an elevated temperature with or without UV radiation. After a set period of digestion time, the resulting CO2 is purged out by a carrier gas for detection. This oxidation technique is more vigorous than UV only even though the digestion time still needs to be optimized for complete oxidation.
(c) Persulfate plus UV irradiation. The sample is simultaneously exposed to persulfate and UV radiation while the resulting CO2 is purged out by a carrier gas. The oxidation is significantly enhanced over UV-only by simultaneous ionization of dissolved organics and the production of highly reactive sulfate free radicals and hydroxyl free radicals.
The CO2 formed from these persulfate oxidation reactions are detected by one of two ways: (1) by allowing it to permeate a membrane into a low conductivity water stream and thereby change the water stream’s conductivity, or (2) by purging the CO2 to an NDIR detector.
The persulfate techniques have the advantage of allowing a large volume of sample, typically between 0.5 and 20 mL to be analyzed, thereby increasing NDIR response to a given concentration. By injecting larger sample volumes, sample response can be increased to easily reach detection limits in the region of 0.002 mg L−1 C.5 Since the majority of system background in this technique is derived from impurity in the persulfate reagent, samples that have low carbon content, below 5 mg L−1 C, can be analyzed with little or no persulfate to optimize the sample to background response ratio yielding the lowest detection limits and the greatest precision.
3 Analytical
There are three TOC analytical parameters that are important to consider when choosing a TOC oxidation technique for various samples. They are sample recovery, detection limits, and particulate analysis. Previous papers on this subject have come to different and contradictory conclusions. However these studies either compared instruments with significantly different flow paths or TOC systems from different eras. Using two modern TOC analyzers with nearly identical flow paths, one using the UV persulfate technique and the other using the HTC technique, the analytical differences and similarities were identified between these two widely used TOC oxidation techniques. Fig. 1 is a flow diagram of the modern HTC TOC analyzer used for this paper’s comparison. Fig. 2 is a flow diagram of the UV-persulfate TOC analyzer also used for this paper.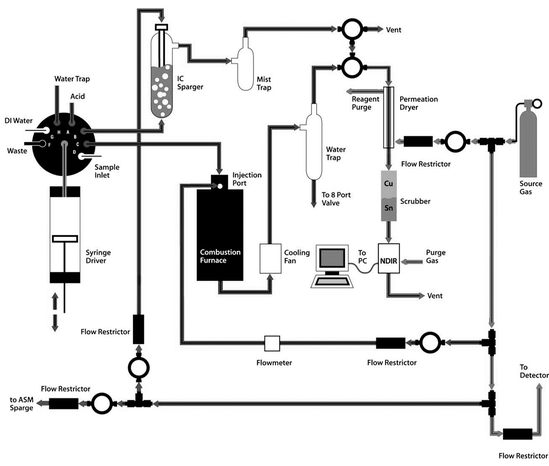 | ||
| Fig. 1 The Tekmar-Dohrmann Apollo 9000HS is an example of a modern HTC TOC analyzer’s flow path.6 | ||
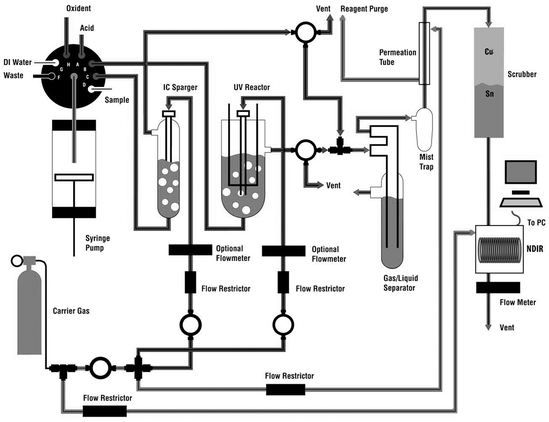 | ||
| Fig. 2 The Tekmar-Dohrmann Phoenix 8000 gives an example of a modern UV-persulfate TOC analyzer’s flow path.5 | ||
3.1 Sample recovery
Sample recovery for persulfate and HTC has been a subject of debate for the past 15 years. Many studies have compared persulfate against HTC using different “tough to oxidize” compounds with conflicting results. Some of the issues that complicate making an accurate comparison include the following.(a) Some studies compare TOC instruments with significantly different flow paths. This brings into question whether poor results are achieved because of oxidation technology or simply poor implementation of that technology.
(b) Some older studies compare TOC instruments from the 1970s–1980s that are not up to date with advances in persulfate and HTC oxidation technology.7,8
(c) Humic acid is often used as a key compound for recovery studies. However, making and maintaining accurate humic acid standards is very difficult due to the nature of the sample matrix. Humic acid is insoluble when the pH ≤ 2. Therefore, it is believed that humic acid becomes colloidal and either precipitates and settles, or flocculates and floats.9,10 For HTC analysis, the humic acid sample was acidified and sparged to remove IC in the sample vial. The sample was also stirred before being injected into the combustion tube to include insoluble forms of humic acid. For the UV-persulfate analysis, the humic acid sample was acidified and sparged to remove IC in the UV reactor with the UV lamp off. After IC removal was complete, the UV lamp was turned on and persulfate reagent was added to oxidize the organic carbon. This process was followed to include insoluble forms of humic acid. Similar benefits of extending the length of the oxidation process to increase the oxidation efficiency for humic acids was found by Kaplan.11
Both techniques gave reasonably good agreement across many different difficult to oxidize compounds as seen in the data in Table 4. UV-persulfate showed better precision on average compared to HTC.
| Compound (n = 3 replicates) | Apollo 9000HS (HTC) | Phoenix 8000 (UV-persulfate)a | ||
|---|---|---|---|---|
| Result/mg1 L−1 C | %RSD | Result/mg L−1 C | %RSD | |
| a For humic acid, IC removal was performed in the UV chamber with the UV lamp off, to include insoluble forms of humic acid which appear when the pH ≤ 2. b Sodium salt of humic acid, Aldrich Chemical Company; assayed by CHNO&S analyzer, 40.34% carbon. | ||||
| Humic acidb | 10.02 | 2.01 | 9.93 | 0.95 |
| Isonicotinic acid | 9.750 | 2.75 | 9.92 | 0.06 |
| Sodium hexane-1-sulfonate | 9.840 | 0.84 | 9.73 | 0.22 |
| Glutaric acid | 9.660 | 0.77 | 9.78 | 0.33 |
| Citric acid | 10.05 | 1.41 | 9.81 | 0.65 |
| Lignosulfonic acid | 9.550 | 0.48 | 9.47 | 0.18 |
| L-Tryptophan | 9.640 | 2.44 | 9.70 | 1.45 |
| 1,4-Benzoquinone | 10.08 | 2.70 | 9.92 | 0.96 |
| Lauric acid | 10.21 | 2.82 | 9.56 | 1.52 |
| Nicotinic acid | 10.05 | 2.35 | 9.94 | 1.29 |
| Average recovery | 98.85% | 1.86 | 97.76% | 0.76 |
3.2 Detection limits
Consideration of detection limits is important for low level TOC measurements, particularly of drinking, ground and sea waters. An instrument detection limit not only establishes how low a level of TOC in a sample can be quantified, it is also an indirect indicator of the ease at which low level TOC in samples can be measured with good accuracy and precision.Detection limits are determined by the ratio of sample TOC response to TOC background for a given TOC instrument. Instrument background, often referred to as the instrument blank, can be broken down into elements that are common to almost all TOC instrument and elements that are specific to a particular oxidation technology. General background elements include: the rinse water used to clean the sample pathways between samples, the TOC derived from the surfaces encountered in the sample pathway either from the material itself (which is often Teflon-based) or though permeation of that material by CO2 in the atmosphere, and any TOC contribution from the acid that is often used in the IC removal step of the TOC process.
Persulfate analyzers have additional TOC background from the persulfate used in the oxidation process. This element can be reduced for low level TOC analysis by cutting the amount of reagents used in the process. For UV based instruments the persulfate can be eliminated for ultra-low level TOC measurements.
HTC analyzers have additional TOC background from the catalyst used and from sample residue buildup in the combustion furnace. Standard Method 5310 states: “The high-temperature methods accumulate nonvolatile residues in the analyzer, whereas, in Method C (The Persulfate-Ultraviolet or Heated-Persulfate Oxidation Method), residuals are drained from the analyzer. Method C (The Persulfate-Ultraviolet or Heated-Persulfate Oxidation Method) generally provides better sensitivity for lower-level (<1 mg L−1) samples.”4 This accumulating effect on the blank for the HTC method of detection can result in a shifting blank, as seen in Fig. 3. While in comparison UV-persulfate provides a consistent blank and low level standard response, as seen in Fig. 4.
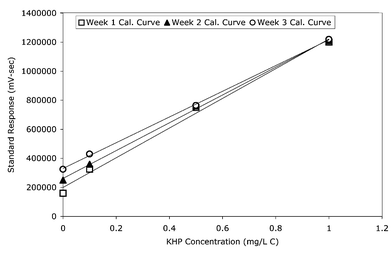 | ||
| Fig. 3 Three low level HTC calibration curves were run over 1 month. Three standards (1, 0.5, and 0.1 ppmC) and a reagent water blank were used to calibrate the HTC analyzer. Squares represent a calibration curve run during week 1. Triangles represent a curve run during week 2. Circles represent a curve run during week 3. This figure illustrates the carry-over factor of residual residues on the blank and low level standards. | ||
 | ||
| Fig. 4 Three low level UV-persulfate calibration curves were run over 1 month. Three standards (1, 0.5, and 0.1 ppmC) and a reagent water blank were used to calibrate the HTC analyzer. Squares represent a calibration curve run during week 1. Triangles represent a curve run during week 2. Circles represent a curve run during week 3. This figure illustrates the low level accuracy over time that UV-persulfate provides for the blank and low level standards. | ||
One way to minimize the effects of TOC background is to increase the amount of sample used in the analysis. The persulfate technique can analyze up to 20 mL of sample per replicate. The increase in sample response improves the sample to background ratio resulting in lower detection limits. The HTC technique can also employ this technique but the maximum sample injection is typically limited to 2 mL, on account of a cooling effect on the catalyst and combustion tube at volumes greater than 2 mL. A comparison of low level TOC standard analysis in Fig. 5 clearly shows the relationship of sample to background response for both persulfate and HTC oxidation techniques.
 | ||
| Fig. 5 System carbon background as a proportion of sample counts for UV-persulfate and HTC oxidation of a 0.100 mg L−1 C potassium biphthalate (KHP) standard. On average, the HTC carbon background is equal to more than 50% of the area counts of a 0.100 mg L−1 C KHP standard. While, the UV-persulfate method background is equivalent to less than 25% of the area counts of the 0.100 mg L−1 C KHP standard. The ability of the UV-persulfate method to remove persulfate from the reaction chamber produces a lower, more consistent blank, as seen with the ratio of the background (reagent water) to sample raw data. | ||
Many HTC manufacturers have recognized instrument blanks as a problem and have recommended options like high-sensitivity catalysts and special procedures, such as lengthy blank checking procedures, to lessen the instrument blank. Even with a high-sensitivity catalyst, the ability for persulfate analyzers to increase sample volume and cut persulfate background contribution results in lower detection limits for persulfate instruments versus the HTC and ultimately better accuracy and precision as shown in Table 5.
| Sample ID (n = 4 replicates) | Apollo 9000HS (HTC) | Phoenix 8000 (UV-persulfate) | ||
|---|---|---|---|---|
| TOC result/mg L−1 C | Standard deviation/mg L−1 C | TOC result/mg L−1 C | Standard deviation/mg L−1 C | |
| 1.00 mg L−1 C KHP | 1.000 | 0.035 | 1.000 | 0.002 |
| 0.50 mg L−1 C KHP | 0.506 | 0.010 | 0.496 | 0.004 |
| 0.25 mg L−1 C KHP | 0.251 | 0.014 | 0.248 | 0.003 |
| 0.10 mg L−1 C KHP | 0.098 | 0.009 | 0.100 | 0.002 |
| 0.05 mg L−1 C KHP | 0.043 | 0.016 | 0.048 | 0.001 |
| Reagent water | 0.012 | 0.013 | 0.007 | 0.001 |
This is not to say low level TOC analysis cannot be done with a high temperature combustion TOC analyzer, the results in Table 5 clearly show low level TOC analysis is possible with HTC. However, as the standard deviations show the results with the persulfate system are much more precise. This increased precision is important because not only does it imply lower detection limits but it is a characteristic that implies stable TOC response over time. It is the superior stability at low levels that makes persulfate systems easier to run and maintain than HTC systems for potable drinking waters or any TOC application below 1 ppmC.
3.3 Particulates
One of the most challenging matrices to analyze is a particulate matrix, which is commonplace in wastewater sites. The heterogeneous nature of particulate samples can cause results with poor reproducibility and accuracy, and in the worst cases, the clogging of lines and valves in contact with the sample. This concern is minimized by letting the sample pass through a filter, for dissolved organic carbon (DOC), as stated in Standard Method 5310B and C. However, many countries in the European community, concerned about particulate organic matter, do not allow sample filtering. In these countries, ISO method 8245![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and EN method 1484
12 and EN method 1484![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 are in place to validate an instrument’s ability to measure samples with particulates.
13 are in place to validate an instrument’s ability to measure samples with particulates.
It is generally accepted that the persulfate oxidation technique gives low recoveries on particulate organic matter above 0.2 mm in size, as stated in EPA method 415.2.14 Consequently, this technique reports DOC values. Many analytical communities use this value as TOC depending on the nature of the sample, such as drinking and pharmaceutical waters. The HTC technique can measure particulate organic matter with attention to instrument sampling techniques. Lee-Alvarez15 reported that a number of factors have an extremely important role in the accurate measurement of TOC with particulate organic matter. A few of these factors are instrument sample handling, sample stirring (gas versus mechanical), and sample volume injected.
In a recent study of natural waters, Najm et al.16 found that in higher turbidity levels of 16 ntu (nephlometric units), TOC results from HTC analyzers yield as much as 40% higher recovery than UV-persulfate. The effects from sample turbidity were determined to be related to particulate organic carbon rather than particulate inorganic carbon. Wei et al.8 also found a similar trend as much as 20% higher recovery for TOC results with HTC compared with UV-persulfate on samples with turbidity levels of 16 ntu. Najm et al.16 also studied the relationship between TOC and DOC as a predictor of DBP formation. Their findings showed a significant relationship between total trihalomethanes (TTHMs) to DOC, after a 24 h chlorination period, of 20–42 µg mg−1, whereas, the ratio of TTHMs to TOC was less than 5 µg mg−1. It was theorized that a portion of TOC is leached into water during the chlorination. They concluded that the reaction between disinfectants and leached DOC actually formed TTHMs and haloacetic acids rather than TOC.16
In most drinking water utilities, particles are removed during the treatment process.17 The turbidity of the settled water is usually less than 1–2 ntu.16 Therefore, presumably DOC is measured at the point of disinfection rather than TOC. Consequently, DOC, either filtered in the laboratory or by particulate settling in the treatment process, is the comparison that is most actively used. As described in previous studies, by decreasing DOC concentrations through treatment, the reduction of disinfectant byproduct formation is most likely to be obtained.
4 Real world samples
Conflicting views have challenged the opinion that both persulfate and HTC are acceptable for natural organic matter determination in surface/drinking water. The industry’s assumption of the two oxidation techniques is that HTC oxidizes TOC better than persulfate in particulate samples.8,16 For samples containing particles greater than 0.2 mm, this may be true; however, some recent studies have hinted that DOC analysis may be a more appropriate surrogate for DBP precursors than TOC.16,18 Still another study indicates that HTC yields significantly lower DOC concentrations than persulfate when inorganic carbon removal conditions are not optimal.19The UV-persulfate and HTC oxidation techniques for DOC and TOC in surface/drinking purification waters were used for analysis, as seen in Table 6. For DOC analysis, each sample was filtered with a Millipore Stericup™ vacuum-driven, disposable filtration system with 0.45 µm HV Durapore™ membrane. Each filter was rinsed with warm (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C) reagent grade water (TOC < 0.050 mg L−1 C) before use. One TOC analyzer used HTC oxidation while the other used UV-promoted persulfate oxidation. Both TOC analyzers had the same flow paths, sample preparation and introduction systems, and detection, as seen in Fig. 1 and Fig. 2. The experimental design is to present an accurate comparison of real world samples for TOC and DOC for the two-oxidation techniques by
eliminating differing flow pathways and detection schemes. Both techniques used the most recent technology advancements as well as optimal instrumentation conditions. Blanks for both TOC analyzers were subtracted in the software after an initial measurement of the instrument’s total blank response given in area counts (mV s).
°C) reagent grade water (TOC < 0.050 mg L−1 C) before use. One TOC analyzer used HTC oxidation while the other used UV-promoted persulfate oxidation. Both TOC analyzers had the same flow paths, sample preparation and introduction systems, and detection, as seen in Fig. 1 and Fig. 2. The experimental design is to present an accurate comparison of real world samples for TOC and DOC for the two-oxidation techniques by
eliminating differing flow pathways and detection schemes. Both techniques used the most recent technology advancements as well as optimal instrumentation conditions. Blanks for both TOC analyzers were subtracted in the software after an initial measurement of the instrument’s total blank response given in area counts (mV s).
| Sample type | Phoenix 8000 (UV-persulfate) | Apollo 9000HS (HTC) | % Difference of persulfate to HTC | |||
|---|---|---|---|---|---|---|
| mg L−1 C | %RSD | mg L−1 C | %RSD | (UV-persulfate/HTC) | ||
| DOC— | ||||||
| Escondido, CA | Distribution | 2.66 | 0.20 | 2.76 | 1.93 | 97 |
| Combination effluent | 2.62 | 0.97 | 2.72 | 0.98 | 96 | |
| Sediment basin | 2.69 | 1.33 | 2.62 | 1.04 | 103 | |
| Flocculation basin | 3.69 | 0.54 | 3.57 | 1.01 | 103 | |
| Lake Wohlford | 5.42 | 0.40 | 5.68 | 0.91 | 96 | |
| Lake Dixon | 2.94 | 0.82 | 2.96 | 1.57 | 100 | |
| Influent | 3.47 | 0.77 | 3.49 | 0.43 | 99 | |
| Brick utility, NJ | Blank | 0.028 | n/a | 0.0312 | n/a | n/a |
| Distribution chamber | 4.9093 | 0.30 | 5.1333 | 2.45 | 96 | |
| Weir 3 | 2.1645 | 0.33 | 2.1718 | 2.06 | 100 | |
| Weir 1 | 2.7131 | 0.98 | 2.8705 | 1.57 | 95 | |
| Point of entry | 2.6883 | 1.01 | 2.7999 | 1.55 | 96 | |
| Intake/river | 4.7272 | 0.56 | 5.0474 | 2.10 | 94 | |
| Passaic Valley, NJ | Filter | 2.1789 | 0.23 | 2.2703 | 1.12 | 96 |
| 100 | 4.4705 | 0.40 | 4.7922 | 1.57 | 93 | |
| Cincinnati, OH | Lake water | 4.502 | 0.38 | 4.724 | 2.29 | 95 |
| River water | 4.842 | 0.53 | 5.046 | 1.87 | 96 | |
| Avg. | 0.61 | n/a | 1.53 | 97 | ||
| TOC— | ||||||
| Cincinnati, OH | Lake water | 5.769 | 0.77 | 6.094 | 3.16 | 95 |
| River water | 5.494 | 0.93 | 5.614 | 2.35 | 98 | |
| Passaic Valley, NJ | Basin 3 and 4 | 2.4393 | 0.40 | 2.5046 | 2.72 | 97 |
| Wanaque North | 2.1506 | 0.34 | 2.2876 | 4.02 | 94 | |
| LFWTP | 2.1853 | 0.16 | 2.2402 | 1.31 | 98 | |
| Settling tube boiler | 17.7839 | 0.81 | 18.5217 | 3.06 | 96 | |
| Greenville, NC | Filtered | 4.2140 | 0.37 | 4.1636 | 1.47 | 101 |
| Raw | 8.6165 | 0.35 | 8.2794 | 1.60 | 104 | |
| Pre-settled 1 | 9.6485 | 1.08 | 9.4384 | 0.72 | 102 | |
| Pre-settled 2 | 9.6698 | 0.96 | 9.7466 | 1.81 | 99 | |
| Avg. | 0.62 | n/a | 2.22 | 98 | ||
A variety of locations across the US were used in this comparison. For real world samples, the results were very clear. Table 6 demonstrates the similarities between persulfate and HTC oxidation for drinking/surface waters. The analysis found that HTC and UV-persulfate yielded a difference of less than 3% for DOC and 2% for TOC on average for drinking/surface water samples. This relationship is typically seen for DOC analysis of drinking water purification plants. Blanks were lower and more consistent with the UV-persulfate oxidation. The shifting blank values associated with the HTC technique may have caused the variance in some of the high and low recoveries, even though they were minimal. UV-persulfate proved better precision overall compared with HTC.
Cincinnati Lake water showed lower DOC results for both methodologies compared to Cincinnati River water. However, for TOC analysis, Cincinnati Lake water exhibited higher TOC results compared to Cincinnati River water on both methodologies. This may point to the fact that Cincinnati Lake water sample contained more particulates than Cincinnati River water. Lakes may have a larger population of microorganisms and more soil and plant matter because of their stagnant waters. Lake water showed a difference of 1.267 ppmC with UV-persulfate and 1.37 ppmC with HTC, with the higher result for TOC as compared with DOC of the same sample. River water showed a difference of 0.652 ppmC with UV-persulfate and 0.568 ppmC with HTC, the higher result being for TOC as compared with DOC of the same sample. These results strengthen the argument made earlier with the difficult to oxidize samples; both methodologies produced similar trends and results for DOC and TOC analysis.
There was a direct comparison between the UV-persulfate and HTC in the surface water samples studied. A linear regression of the HTC and UV-persulfate for DOC sample results found a correlation coefficient of 0.995, as seen in Fig. 6. This relationship complements previous findings between oxidation techniques for DOC analysis.11,20 A linear regression of the HTC and UV-persulfate for TOC sample results found a correlation coefficient of 0.997, as seen in Fig. 7. These results contrasted some earlier findings for TOC analysis of freshwater samples. However, these samples were sampled from actual drinking water purification utilities and were analyzed on instrumentation whose only difference was the oxidation technique.
 | ||
| Fig. 6 Linear regression of HTC versus UV-persulfate DOC results. | ||
 | ||
| Fig. 7 Linear regression of HTC versus UV-persulfate TOC results. | ||
For many drinking water purification plants, TOC samples are taken from different segments of their treatment process. A majority of the particulates in the source water settle during treatment. Therefore, typical drinking water laboratories perform DOC analysis as per their standard operation procedures. When samples are not filtered, it is assumed that the particulates have typically settled out or have partially dissolved in holding treatment tanks before being sampled.
5 Conclusions
Overall, both TOC oxidation technologies demonstrated comparable results for TOC analysis of potable water. Analytical parameters such as sample recovery, detection limits, and particulates should be considered when determining which oxidation technique is bested suited for a water type. Sample recovery for both techniques was comparable (<2–3% difference) for both TOC and DOC surface water samples. Low level TOC accuracy and precision was superior with the persulfate technique as a result of favorable sample instrument background ratios versus the HTC technique. Although not studied in this paper, future research should be directed towards the relationship between DBPs, such as trihalomethanes and haloacetic acids, and their precursors. Most notably, determining if there is a preference between TOC or DOC for predicting DBP formation.Acknowledgements
The author is grateful to Brick Utilities (Brick Twp., NJ), City of Escondido Water Plant, Greenville Utilities Commission and Passaic Valley Water Laboratory (Totowa, NJ) for assisting with the procurement of samples. Also, the author thanks Susan Griffiths-Hoffman for help with graphical design.Millipore Elix™ and Milli-Q Gradient™ Water Polishing System was used for production of all reagent grade water used in preparing TOC standards. Millipore Stericup™ Vacuum Disposable Filtration System with 0.45 µm HV Durapore™ membranes were used in filtering samples when indicated for DOC analysis.
References
- J. O. Zavaleta, F. S. Hauchman and M. W. Cox, in Formulation and Control of Disinfection Byproducts in Drinking Water, ed. P.C. Singer, AWWA, Denver, CO, 1999, ch. 5, and references cited therein Search PubMed.
- EPA, Fed. Regist., 1998, 63, 69390 Search PubMed.
- EPA, Fed. Regist., 1998, 63, 69478 Search PubMed.
- Standard Methods for the Examination of Water and Wastewater, ed. A. D. Eaton, L. S. Clesceri and A. E.Greenberg., APHA/AWWA/WEF, Washington, DC, 19th edn. Supplement, 1996, Method 5310 Search PubMed.
- Phoenix 8000™ User Manual, Rev. C, Tekmar-Dohrmann, Mason, OH, 1998 Search PubMed.
- Apollo 9000HS™ User Manual, Rev. C, Tekmar-Dohrmann, Mason, OH, 2000 Search PubMed.
- J.-F. Koprivnjak, J. G. Blanchette, R. A. Bourbonniere, T. A. Clair, A. Heyes, K. R. Lum, R. McCrea and T. R. Moore, Water Res., 1995, 29, 91 CrossRef CAS.
- Y. Wei, J. Reavis, C. Meadows, L. Woung and J. Greenlee, Proc. Water Qual. Technol. Conf., AWWA, San Diego, CA, 1998, 2-E3 (on CD-ROM) Search PubMed.
- D. Langmuir, Aqueous Environmental Geochemistry, Prentice-Hall, Upper Saddle River, NJ, 1992, pp. 161–162. Search PubMed.
- S. E. Manahan, Environmental Chemistry, Lewis/CRC, Boca Raton, FL, 6th edn., 1994, pp. 80–82 Search PubMed.
- L. A. Kaplan, Limnol. Oceanogr., 1992, 37, 1119 Search PubMed.
- Water quality – Guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC), International Organization for Standardization, Geneva, 1999, ISO method 8245 Search PubMed.
- Water analysis – Guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC), European Committee for Standardization, Brussels, 1997, EN method 1484 Search PubMed.
- Methods for Chemical Analysis of Water and Wastes, US EPA Environmental Monitoring and Support Laboratory, Cincinnati, OH, 1983, EPA-600/4-79-020, Method 415.2 Search PubMed.
- M. T. Lee-Alvarez, Total Organic Carbon Analysis of Particulated Samples, Application Note Vol. 9.19, Tekmar-Dohrmann, Mason, OH, 1999 Search PubMed.
- I. Najm, J. Marcinko and J. Oppenheimer, J. Am. Water Works Assoc., 2000, 92(8), 84 Search PubMed.
- K. Ruehl, Water Eng. Manage., 1999, 146, 22 Search PubMed.
- E. T. Urbansky, J. Environ. Monit., 2001, 3, 102 RSC.
- L. A. Kaplan, J. Am. Water Works Assoc., 2000, 92(4), 4, 149 Search PubMed.
- R. Benner and J. I. Hedges, Mar. Chem., 1993, 41, 161 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |
