The role of size exclusion chromatography–flame atomic absorption spectrometry in the treatment chemistry of potable water†
Marina C.
Koether
Department of Chemistry and Biochemistry, Kennesaw State University, 1000 Chastain Road, Kennesaw, GA 30144, USA
First published on 11th October 2001
Abstract
The percentage composition of Al13, [AlO4Al12(OH)24(H2O)12]7+, in water treatment coagulants is an important criterion in the development and use of polymeric coagulants. Polymeric coagulants are generally used in cold climates or with highly turbid waters. Size exclusion chromatography–flame atomic absorption spectrometry (SEC-FAAS) can separate Al13 and monomeric Al within 6 min. The percentage composition of Al13 and monomeric Al is determined by solving two simultaneous equations. Due to overlapping peaks, a 10% error is associated with this method of quantification. This method can be used on coagulants of varying “r values” (r = [OH−]/[Al3+]), or on mixtures of those coagulants and monomeric aluminium.
 Marina C. Koether Marina C. Koether |
Introduction
Both monomeric and polymeric aluminium species may be used as coagulating agents in water treatment. A fast and easy method for their identification and measurement is necessary for optimizing their use. Developmental steps in their analyses are provided by examining water treatment, aluminium coagulants, size exclusion chromatography (SEC) and flame atomic absorption spectrometry (FAAS).Water treatment
The production of potable water most often involves clarification beyond what is achieved by sedimentation. This clarification is in the form of coagulation, flocculation and filtration. Coagulation and flocculation occur operationally together. These processes begin with coagulant addition and end with the sedimentation prior to sand filtration. Coagulation eliminates repulsion between colloidal particles while flocculation involves the formation of flocs due to the coming together of the destabilized colloidal particles. Typically, coagulation occurs immediately upon the addition of the coagulant to a rapidly mixed stream of raw water. Flocculation takes place by slowly stirring the coagulated water.1Bench-scale and pilot-plant studies are used to determine the optimum treatment conditions based on the water quality. The conventional jar-test is the simplest method for determining the optimum treatment conditions. It involves the coagulation, flocculation and sedimentation steps of water treatment, but not the filtration step. The procedure involves adding coagulants and other coagulant aids to the rapidly mixing raw water and allowing sufficient time for coagulation. The water is then slowly mixed for a set period of time to cause flocculation and is subsequently allowed to settle before measurements of residual turbidity, color, Al, etc. are made.
Two mechanisms of coagulation are predominant in the literature, and the choice of coagulant often depends on the method that applies to the particular type of raw water. The two mechanisms are charge neutralization (via compression of the double layer and adsorption to produce charge neutralization) and sweep floc (via enmeshment in a precipitate and adsorption to permit interparticle bridging). When Al salts are used as coagulants, effective coagulation occurs in the pH range of 6.5 to 8.5 where the solubility of the hydrolysis products of Al is at a minimum. If the pH of the raw water or treated water is lower or higher, pH adjustments are made to be within the pH range for effective coagulation and to avoid corrosion in the distribution lines. The metal salts hydrolyze in water and react with species in the water to form hydrolytic metal complexes. These species are essential for coagulation, but are only intermediate species during the coagulation, and they ultimately form the insoluble (hydr)oxides.1
Aluminium coagulants
Aluminium may exist as soluble monomers and polymers, form complexes with a variety of organic and inorganic ligands and be present as insoluble amorphous or crystalline Al species. The monomeric hydrolytic Al species include the hexa-aquo cation, Al(H2O)63+; the monohydroxide cation, AlOH(H2O)52+; the dihydroxide cation, Al(OH)2(H2O)4+; aluminum hydroxide, Al(OH)3 and the aluminate ion, Al(OH)4−. Although many polymeric hydrolytic Al species have been proposed, only two have been verified to exist in solution and the solid state. These are the dimer [Al2(OH)2]4+ and tridecamer (Al13), [AlO4Al12(OH)24(H2O)12]7+. In Al13, twelve octahedrally coordinated Al atoms in a cage-like structure surround the tetrahedral Al. The Al13 structure may be viewed as six oxygen-sharing dimeric units, which bridge the edges of the central tetrahedron.2The Al13 cation is produced in the laboratory by adding a base, such as a carbonate or hydroxide, to an acidic Al salt solution. If the ratio of OH− to Al3+ (r = [OH−]/[Al3+]) is less than 3, then the resulting solutions are considered partially neutralized Al solutions (PNAS). The characteristics of the PNAS are dependent on the mode of preparation, such as the temperature, rate of stirring and choice of reactants. These characteristics change with age, dilution, or thermal treatment.3 The r value is one factor that determines the amount of monomeric and polymeric species present. As the r value increases in PNAS, the percentage of monomeric Al decreases almost linearly and the amount of polymeric species, in particular the Al13 species, increases up to r = 2.5. Due to the specific conditions required for the synthesis of Al13, [AlO4Al12(OH)24(H2O)12]7+, it is probably not a hydrolysis product formed during the conventional water treatment process. Thus, if Al13 is to be used as a coagulating agent, it must be present in the coagulant prior to addition to the raw water.
The most common commercially available polymeric Al coagulant is PAC (poly-aluminium-chloride). In most cases, PAC has an r value of 1.5. Other formulations called PAS (poly-aluminium-sulfate) and PAHS (poly-aluminium-hydroxy-sulfate) are not commercially available but also have an r value of 1.5. PAHS can be made at the plant by adding powdered limestone to a stirred solution of alum. The percentage of Al present as Al13 is only 10%. The production and use of PAHS only adds 10% to the cost and yet more than a 10% improvement in water quality is observed in most cases studied.4–6
Solutions containing about 95% Al13 (r = 2.5) can be synthesized and have been shown to be an effective and superior coagulant to alum under certain conditions.7 Equilibrium solubility diagrams are often used to describe the species present during coagulation. However, equilibrium is not achieved during the water treatment process for alum. This indicates the reason for the poor results for alum in cold waters where the hydrolysis is slow. Al13 is effective in both warm and cold waters since the Al13 species bypasses the need for hydrolysis.
Cost can be a prohibitive factor for the use of about 95% Al13 solutions. In addition, the toxicity of Al13 is in question.2 Thus, it is proposed that solutions containing Al13 be combined with monomeric Al in ratios and in sequences that are best suited for the raw water quality parameters. The Al13 and monomeric Al percentage composition of these preparations and the commercially available coagulants can be determined through size exclusion chromatography-flame atomic absorption spectrometry (SEC-FAAS).
SEC
SEC separates molecules according to their size as they pass through a column. SEC is most frequently used for the separation of polymers and biological molecules according to their molecular size. In the present study, the same technique has been applied to hydrolyzed Al salt solutions to determine the presence of polymeric species. The SEC column is filled with a bead-formed gel, prepared by cross-linking dextran with epichlorohydrin. The degree of swelling and pore size are dependent on the degree of cross-linking. Therefore, the pores of the gel have a carefully controlled range of sizes and are filled by the liquid mobile phase. The analyte fractionation range, based on the molecular weight of dextrans and globular proteins, is dependent on the pore size. Since mixing and diffusion occurs, the size-excluded components appear in Gaussian-shaped bands. Previous reports8 of partially neutralized aluminium chloride solutions studied by SEC suggest that polymeric Al would elute first followed by monomeric Al.FAAS
FAAS quantifies trace metals in aqueous samples. A nebulizer converts the sample into a fine mist in a spray chamber where it mixes with nitrous oxide (for Al) and acetylene and then passes to the burner where the flame’s thermal energy desolvates the aerosol mist, volatilizes the particles and produces free atoms. The free atoms absorb radiation from a hollow cathode lamp and the amount of absorption is related to standards to determine the concentration of the element in the original solution. The flow rate/suction by nebulization of the aqueous solution must remain constant to provide quantitative data.Aim of investigation
The percentage composition of Al13 in coagulants is an important criterion in the development and use of polymeric coagulants. The composition of a mixture of the two active ingredients, monomeric Al and Al13, in coagulants could be adjusted based on the needs of the raw water conditions. It is advantageous to be able to separate and measure Al13 and monomeric Al content in the coagulants. The coupling of SEC to FAAS provides a rapid method of analysis for such determinations.Description of the experimental procedures
SEC was carried out using a C16/40 column (Pharmacia LKB Biotechnology) with an internal diameter and length of 1.6 and 40 cm, respectively. Prior to packing, 38.5 g of Sephadex™ G-10 (Pharmacia LKB Biotechnology) was allowed to swell in an excess amount of de-aerated, filtered 0.01 M HNO3. The column was packed to a gel height of 36 cm by gravity feed. Sephadex G-10 has the lowest fractionation range possible with fractionation up to 700 MW in terms of proteins. Al3+ would travel the longest distance and Al13 would travel the shortest distance. The mobile phase was 0.1% KCl in 0.01 M HNO3 using degassed, deionized water. A peristaltic pump maintained a flow rate of 10 mL min−1. The sample loop was approximately 250 µL. The tubing leaving the bottom of the column was attached to the suction tubing of the FAAS instrument such that the distance was minimal. Due to the close proximity of the column to the flame, the thermostat jacket provided temperature control and stability by using circulated temperature-controlled water set at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
°C.
A Perkin Elmer AAS 4000 was used to measure Al at 309.3 nm using an Al hollow cathode lamp and a 0.7 nm slit-width, 4 cm slit nitrous oxide–acetylene flame and AA mode only. The data acquisition program was written in Borland C. The output voltage (0 to 1 V) was collected versus time using a CIO-DAS16/Jr/16 data acquisition board, a CIO-MINI37 universal screw terminal and a C37FFS-5 shielded cable. The data were displayed on a computer and saved as XY (time and absorbance) data and uploaded into Excel for analysis.
The preparation of polymeric Al solutions containing about 95% Al13 involves heating 30 mL of 1.67 M AlCl3 (hydrate from Fisher) to 80 °C, adding 50 mL of 1.25 M Na2CO3 (Fisher) and quantitatively transferring the solution to a 100 mL volumetric flask to achieve a final concentration of 0.501 M Al and an r value of 2.5. During these experiments, the r = 2.5 solution produced solids that did not redissolve. This would indicate that the concentration of aluminium was less than indicated, as solids are formed and remain when r > 2.5. Thus, the r = 2 solutions were used representing about 95% Al13. Further dilution of 20 mL to 100 mL with the mobile phase is required immediately before SEC-FAAS. The maximum concentration measured is 0.10 M Al. The samples were also measured by FAAS in the continuous mode, separate from the column, by a further dilution of 2 mL into 100 mL in order to quantify the total Al. These solutions were 54 ppm in Al. Calibration standards were made from Fisher 1000 ppm Al solution with the maximum at 50 ppm Al.
Samples of r = 0 are monomeric aluminium solutions. As r increases, more polymeric species are present. Two sets of samples were measured. The unmixed samples studied were of varying r values (0, 1, 1.5, 2). The mixed samples involved mixing solutions of varying r values (1, 1.5, 2) with solutions of r = 0 in various ratios.
Results
Calibration bypassing column
Fig. 1 illustrates the calibration data taken with the column and injection port bypassed. The solution sampling time was sufficient to establish an absorbance response plateau. The samples were analyzed after the standards. The calibration equation of the line is Absave = 0.0046 × CAl,ppm with r2 = 0.99. The unknown solutions “a” were made to be 54 ppm Al. However, there is a decrease in the absorbance with time, decreasing from 51 to 45 ppm Al across the series. The unknown solutions “b”, “c” and “d” represent 40.5, 27 and 13.5 ppm Al, respectively. These data are consistent with an overall 10% error associated with the drift. These data illustrate the precision obtainable with this old instrument.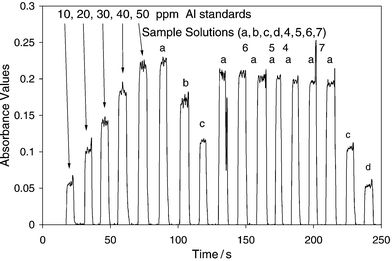 | ||
| Fig. 1 Example of an analysis of aluminium standards and sample solutions using FAAS without SEC. Sample solutions a, b, c and d are 54, 40.5, 27 and 13.5 ppm Al, respectively. For solutions 4, 5, 6, and 7 refer to Table 1. | ||
Calibration from chromatograms
Calibration curves were created from chromatograms using r = 2 and r = 0 solutions of various concentrations (0.100, 0.0500, 0.0250 M Al). The r = 2, 0.100 M Al solution achieved a higher absorbance than the calibration curve data obtained by the column bypass method at the retention time (tr) of 160 s. Thus, the absorbance value for this solution (0.100 M) was only used at a tr of 202 s. Using only the lower concentrations (0.0250 and 0.0500 ppm), a calibration curve was obtained with Absave = 4.38 [Al] at tr = 160 s. The two simultaneous equations obtained are as follows:| abstr = 160s = 4.38[Al]Al13 + 0.0600[Al]monomeric aluminium | (1) |
| abstr = 202s = 0.431[Al]Al13 + 1.31[Al]monomeric aluminium | (2) |
Chromatograms
Figs. 2 and 3 illustrate the chromatograms possible. Only two distinguishable peaks are detected with some overlap between them. Monomeric aluminium elutes last in the broad peak and the Al13 elutes first. The elution of the Al13 occurs very close to the void volume. This is expected as the molecular weight of Al13 is greater than 700, the maximum exclusion range for proteins for the gel, Sephadex G10. The dimers, if present, are considered to elute with the monomeric Al. Using the above data, the concentration of Al13 and monomeric aluminium in the solutions in Figs. 2 and 3 are given in Table 1, based on their absorbance at tr of 160 and 202 s.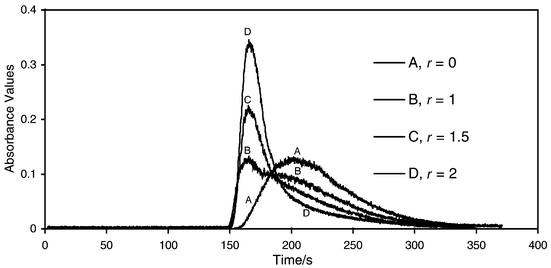 | ||
| Fig. 2 SEC-FAAS analysis of aluminium solutions (0.10 M Al) with various r values. | ||
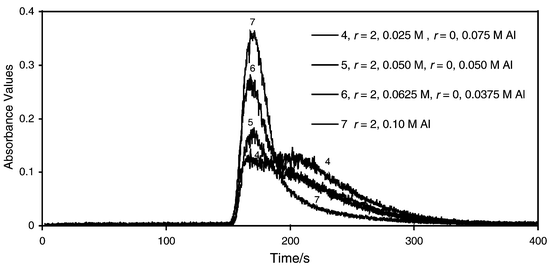 | ||
| Fig. 3 SEC-FAAS analysis of mixtures of aluminium solutions of various r values. | ||
| Solution | Abs (160 s) | Abs (202 s) | [Al13] | [Al3+] | % Al13 |
|---|---|---|---|---|---|
| 1. 0.10 M Al r = 0 | 0.006 | 0.131 | 0 | 0.1 | 0 |
| 2. 0.10 M Al r = 1 | 0.110 | 0.092 | 0.0243 | 0.0622 | 28 |
| 3. 0.10 M Al r = 1.5 | 0.171 | 0.069 | 0.0385 | 0.0401 | 49 |
| 4. 0.025 M Al r = 2 + 0.075 M Al r = 0 | 0.102 | 0.132 | 0.0220 | 0.0935 | 19 |
| 5. 0.050 M Al r = 2 + 0.050 M Al r = 0 | 0.182 | 0.0986 | 0.0407 | 0.0619 | 40 |
| 6. 0.0625 M Al r = 2 + 0.0375 M Al r = 0 | 0.283 | 0.0949 | 0.0639 | 0.0514 | 55 |
| 7. 0.10 M Al r = 2.0 | 0.365 | 0.0626 | 0.0831 | 0.0205 | 80 |
Discussion
The results in Table 1 illustrate that a mixture of a polymeric coagulant with a monomeric coagulant will contain both active ingredients in the proportions desired within experimental error. For example, doubling the polymeric component from 0.025 M Al (solution 4) to 0.050 M Al (solution 5) resulted in a doubling of the concentration of Al13. The 0.10 M Al polymeric solution (solution 7) contains double the amount of Al13 than the 0.050 M Al solution (solution 5). Thus, the SEC-FAAS method can be used to determine the percentage composition of Al13 in a mixture of Al13 and monomeric aluminium.It should be noted that this method is not truly quantitative and that detection limits are not obtained and are not relevant for the purpose of these measurements. The day-to-day fluctuations and drift observed during each analysis causes poor precision and results, approximately 10% error in the quantitative results. As seen in Figs. 2 and 3, the r = 2 solution has a tail on its elution peak that interferes with the monomeric peak. This overlap increases the uncertainty of the measurement. However, by examining the shape of the peaks, the percentage composition as found in Table 1 can be expected. Peak heights at the two retention times were used in solving the two simultaneous equations to calculate the concentrations of the two substances in the mixtures. This method is user-friendly for small utilities using relatively old FAAS instrumentation for this work. The cost of such a setup is minimal due to the possible use of a strip chart recorder with the older FAAS instruments rather than the computer interface used here.
The results in Table 1 also illustrate the percentage Al13 created in the r = 1 and r = 1.5 solutions. These values are high when compared to those found in PAC and PAHS. Those solutions only contain 10% of the aluminium as Al13. The increased amount is probably due to the heating involved in the preparation of these solutions. It would be more advisable to mix monomeric solutions with the polymeric solution to maximize the effectiveness and minimize cost.
Fig. 1 illustrates the continuous response obtained by FAAS versus the transient responses obtained in the chromatograms in Figs. 2 and 3. Although solution-sampling time was sufficient to establish a steady-state plateau, the plateau had a high amount of scatter and thus the average had a high amount of uncertainty. The mobile phase had a high concentration of KCl, which would deposit over time on the flame head. Periodic cleaning of the flame head was necessary between chromatograms. The instrumentation can be relatively inexpensive since high tech instrumentation (low detection limits) is not necessary for this work. Thus, old instrumentation or field instrumentation is suitable.
Separation and detection of Al13 and monomeric Al is feasible by SEC-FAAS. Chromatograms are completed within 6 min. It is foreseeable that this method may be used in the future, as the use of Al13 as a coagulating agent increases in the water treatment industry. Thus, the future of water treatment in this area involves exploring the applicability of mixtures of Al13 with monomeric Al versus monomeric aluminium and the timing of their application to the water stream due to the different mechanisms involved.
Acknowledgements
The author thanks Dr. Gary vanLoon of Queen’s University and the Departments of Biology, Chemistry and Earth Science at Northeastern Illinois University for the use of the instrumentation. Special thanks goes to Dr. Mike McCann of Sam Houston University for the computer program. The undergraduate students who participated in this project from Northeastern Illinois University were Raesin Caine, Christian Giacolone and Michael Kenny.References
- S. D. Faust and O. M. Aly, Chemistry Of Water Treatment, Ann Arbor Press, Chelsea, MI, USA, 2nd edn, 1998, pp. 215–270 Search PubMed.
- The Environmental Chemistry of Aluminum, ed. G. Sposito, CRC Press, Boca Raton, FL, 1989 Search PubMed.
- P. M. Bertsch, Soil Sci. Soc. Am. J., 1987, 51, 825 CAS.
- M. C. Koether, J. E. Deutschman and G. W. vanLoon, J. Environ. Eng. Div.-ASCE, 1997, 123(9), 859 Search PubMed.
- K. N. Exall and G. W. vanLoon, J. Am. Water Works Assoc., 2000, 92(11), 93 Search PubMed.
- J. Deutschman, K. Exall, G. Lever, J. Mayo and G. vanLoon, Opflow, 2001, 27(4), 8 Search PubMed.
- M. C. Koether and N. Carmosini, Proceedings of the American Water Works Association Annual Conference, Water Research, Toronto, Ontario, June 23–27, 1996, American Water Works Association, Denver, CO, 1996, pp. 99–120 Search PubMed.
- J. W. Akitt and A. Farthing, J. Chem. Soc., Dalton Trans., 1981, 1606 RSC.
Footnote |
| † Electronic Supplementary Information available. See http://www.rsc.org/suppdata/em/b1/b105581J/ |
| This journal is © The Royal Society of Chemistry 2002 |
