Direct thioesterification from carboxylic acids and thiols catalyzed by a Brønsted acid
Shinya
Iimura
,
Kei
Manabe
and
Shū
Kobayashi
*
Graduate School of Pharmaceutical Sciences, The University of Tokyo, CREST, Japan Science and Technology Corporation (JST), Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan. E-mail: skobayas@mol.f.u-tokyo.ac.jp
First published on 21st December 2001
Abstract
In the presence of a catalytic amount of trifluoromethanesulfonic acid, free carboxylic acids reacted with free thiols directly to afford the corresponding thioesters in high yields.
Thioesters are synthetically useful as well as biologically important compounds because of their high reactivity toward various nucleophiles. For the preparation of thioesters, the most popular method is the reaction of acyl chlorides with thiols or the reaction of carboxylic acids with thiols in the presence of a stoichiometric amount of a condensing agent such as 1,3-dicyclohexylcarbodiimide (DCC).1 From the atom-economical2 and environmental points of view, direct thioester formation from free carboxylic acids and free thiols is desirable.3 However, to the best of our knowledge, although there have been a few reports on synthesis of thiolactones by acid-accelerated intramolecular thioesterification,4 there have been no reports on catalytic intermolecular direct thioesterification of carboxylic acids with thiols.5,6 This is probably because equilibrium in the reactions of carboxylic acids with thiols is not favourable for thioester formation, and a large activation barrier exists between the materials (carboxylic acids and thiols) and the products (thioesters).7 In this paper, we report the first example of Brønsted acid-catalyzed intermolecular direct thioesterification of carboxylic acids with thiols, that proceeds smoothly in toluene under azeotoropic reflux conditions.
First, we examined the catalytic activity of several Brønsted acids and Lewis acids (10 mol%) in a model reaction of lauric acid (1.0 equiv) with dodecanethiol (1.0 equiv) in toluene at reflux for 6 h with removal of water (Table 1). As expected, the reaction did not proceed at all without a catalyst (entry 1). This result indicates that direct thioesterification is difficult under simple azeotropic reflux conditions to shift the equilibrium from the materials to the products. On the other hand, it was exciting to find that Brønsted acids were effective for the present reaction (entries 2–6). Among Brønsted acids tested, trifluoromethanesulfonic acid (TfOH) was the most effective in this thioesterification (entry 4). It was interesting to find that Nafion-H was also effective, but that longer reaction time was needed (entry 6).8 It is noteworthy that the TfOH-catalyzed reaction proceeded in 10 mmol-scale without any difficulties, and that only 1 mol% of TfOH was enough to catalyze the reaction to afford the desired thioester in 94% yield (entry 4). On the other hand, when this TfOH-catalyzed reaction was carried out without a solvent at the same temperature, the thioester was obtained in only 43% yield. In addition, Lewis acids such as TiCl4, ZrCl4, HfCl4, NbCl5, and SnCl4 were less active or inert (entries 7–11).
Next, we investigated substrate generality in TfOH-catalyzed direct thioesterification of carboxylic acids with thiols (1∶1) in toluene under azeotropic reflux conditions (Table 2).† The reactions proceeded not only for primary and sterically hindered secondary aliphatic but also for aromatic thiols to give the corresponding thioesters in high to excellent yields (entries 1–4). The reaction also proceeded using various carboxylic acids (entries 5–11). Although sterically crowded and aromatic carboxylic acids were less reactive than linear aliphatic substrates, their reactions proceeded smoothly although their reaction times were longer (entries 7–9). α,β-Unsaturated carboxylic acids reacted smoothly under the conditions (entries 10 and 11). It is noted that equimolar amounts of free carboxylic acids and free thiols reacted directly to afford the corresponding thioesters in high to excellent yields.
|
|
||||
|---|---|---|---|---|
| Entry | R1COOH | R2SH | Time/h | Yield (%) |
| a One mol% of TfOH was used. The reaction time was 12 h. | ||||
| 1 | CH3(CH2)10COOH | CH3(CH2)11SH | 6 | 97 (94)a |
| 2 | CH3(CH2)10COOH | PhCH2SH | 6 | 95 |
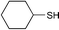
| 3 | CH3(CH2)10COOH |
|
12 | 93 |
| 4 | CH3(CH2)10COOH | PhSH | 6 | 76 |
| 5 | PhCH2CH2COOH | CH3(CH2)11SH | 6 | 93 |
| 6 | PhCH2CH2COOH | PhCH2SH | 6 | 94 |
| 7 |

|
CH3(CH2)11SH | 12 | 96 |
| 8 |

|
CH3(CH2)11SH | 36 | 92 |
| 9 | PhCOOH | CH3(CH2)11SH | 48 | 87 |
| 10 | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOH CHCOOH |
CH3(CH2)11SH | 8 | 76 |
| 11 | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOH CHCOOH |

|
10 | 80 |
In summary, direct thioesterification of carboxylic acids with thiols is efficiently catalyzed by TfOH in toluene under azeotropic reflux conditions. This method provides not only an atom-economical process but also a simple and practical protocol for thioester synthesis.
This work was partially supported by a Grant-in-Aid for Scientific Research from Japan Society of the Promotion of Science.
Notes and references
- O. Jeger, J. Norymberski, S. Szpilfogel and V. Prelog, Helv. Chim. Acta, 1946, 29, 684 CrossRef CAS; J. R. Grunwell and D. L. Foerst, Synth. Commun., 1976, 6, 453 CAS; T. Imamoto, M. Kodera and M. Yokoyama, Synthesis, 1982, 134 CrossRef CAS.
- B. M. Trost, Science, 1991, 254, 1471 CrossRef CAS.
- Recently, direct esterification of carboxylic acids with alcohols has been reported. See: K. Ishihara, S. Ohara and H. Yamamoto, Science, 2000, 290, 1140 Search PubMed; K. Wakasugi, T. Misaki, K. Yamada and Y. Tanabe, Tetrahedron Lett., 2000, 41, 5249 CrossRef CAS; J. Otera, Angew. Chem., Int. Ed., 2001, 40, 2044 CrossRef CAS; K. Manabe, X.-M. Sun and S. Kobayashi, J. Am. Chem. Soc., 2001, 123, 10101 CrossRef CAS.
- P. Catsoulacos and Ch. Camoutsis, J. Heterocycl. Chem., 1976, 13, 1315 Search PubMed; S. Karel and P. Miroslav, CS Patent, 194006; ; Chem. Abstr., 1982, 97, 109888z. Search PubMed.
- J. Voss, Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon, Oxford, 1991, vol. 6, p. 435. Search PubMedIn fact, the difficulty of acid-catalyzed direct thioesterification was stated as follows: ‘Unlike carboxylic esters, open-chained S-alkyl thiocarboxylates cannot be obtained by direct proton-catalyzed esterification’..
- Lipase-catalyzed direct thioesterification was reported. However, this method still lacks substrate generality, and requires rather delicate reaction conditions. See: M. Caussette, A. Marty and D. Combes, J. Chem. Tech. Biotechnol., 1997, 68, 257 Search PubMed; N. Weber, E. Klein, K. Vosmann and K. D. Mukherjee, Biotechnol. Lett., 1998, 20, 687 Search PubMed; N. Weber, E. Klein and K. D. Mukherjee, Appl. Microbiol. Biotechnol., 1999, 51, 401 CrossRef CAS; N. Weber, E. Klein and K. D. Mukherjee, J. Am. Oil Chem. Soc., 1999, 76, 1297 CrossRef CAS.
- T. C. Bruice, Organic Sulphur Compounds, ed. N. Kharasch, Pergamon, London, 1961, vol. 1, p. 421. Search PubMed.
- G. A. Olah, P. S. Iyer and G. K. S. Prakash, Synthesis, 1986, 513 and references therein. Search PubMed.
Footnote |
| † General procedure: A flame-dried, 20 mL, single-necked, round-bottomed flask fitted with a stir bar and a 10 mL pressure-equalized addition funnel (containing a cotton plug and 2 g of 4 Å molecular sieves) surmounted by a reflux condenser was charged with a carboxylic acid (0.5 mmol), a thiol (0.5 mmol), and TfOH (0.05 mmol) in toluene (5 mL). The mixture was brought to reflux with removal of water. After 6–48 h, the resulting mixture was cooled to rt, and an aqueous solution of saturated NaHCO3 was added. The resultant mixture was extracted with ethyl acetate, and the organic layer was dried over anhydrous Na2SO4. The solvents were evaporated, and the residue was purified by preparative TLC on silica gel to give the pure product. |
| This journal is © The Royal Society of Chemistry 2002 |


