Nanotube composites: novel SiO2 coated carbon nanotubes†
T.
Seeger
a,
Th.
Köhler
b,
Th.
Frauenheim
b,
N.
Grobert
c,
M.
Rühle
a,
M.
Terrones
*cd and
G.
Seifert
b
aMax-Planck-Institut für Metallforschung, Seestraße 92, 70174, Stuttgart, Germany
bTheoretische Physik, Universität-GH Paderborn, 33098, Paderborn, Germany
cSchool of Chemistry, Physics and Environmental Science, University of Sussex, Brighton, UK BN1 9QJ. E-mail: mterrones@ipicyt.edu.mx
dIPICYT, Av. Venustiano Carranza 2425-A, Col. Bellas Lomas, San Luis Potosí, 78210, México
First published on 4th December 2001
Abstract
A novel route to nanocomposites consisting of multi-walled carbon nanotubes (MWNTs) embedded in amorphous SiOx is reported; the material has been characterised by high resolution transmission electron microscopy (HRTEM) and high resolution electron energy loss spectroscopy (HREELS); for the first time, and based on our observations, we propose theoretical models acounting for stable SiOx/tube interfaces using density functional based tight binding (DFTB).
Carbon nanotubes are excellent candidates for the fabrication of robust composites1,2 and conducting polymers3,4 due to their fascinating electronic and mechanical properties. The formation of stable tube-matrix interfaces is crucial for composite applications. However, the surface of MWNTs of large diameter (>20 nm OD), which exhibit large Young’s moduli of ca. 1–1.3 TPa,5,6 tends to be similar to graphite, and chemically ‘inert’. Therefore, surface modification treatments are required so that efficient tube–matrix interactions are established. Unfortunately, these treatments can result in the extreme degradation of the tubes and the decrease in the Young’s modulus, which leads to a significantly lower tensile strength of the the composites.7
The creation of stable nanotube coatings, which do not significantly alter the tube surface, is feasable in order to circumvent this problem. These coated nanotubes are expected to exhibit enhanced mechanical properties when compared to heavily degraded nanotubes. From the chemical point of view, MWNTs coated with SiOx reveal a higher oxidation resistance, oxidation being a common drawback of all-carbon materials. Therefore, a detailed study of nanotube SiOx coatings is crucial form the theoretical and experimental viewpoint.
Recent work has addressed the formation of irregular metal oxide coatings on MWNTs using single-step chemical routes at low8,9 and high temperatures.8,10 Here, we report a novel synthesis method to generate bulk nanocomposites consisting of MWNTs embedded in, and coated with SiO2. The materials were prepared by combining a sol–gel technique with a sintering process at high temperatures. For the first time, we have studied the tube/matrix interfaces using HRTEM, HREELS and DFTB calculations.11
The MWNT/SiOx bulk composite was produced as follows: a mixture of 250 mg MWNTs (arc discharge tubes12) with 5 g H2O (pH 2 adjusted with HCl) and 10 g TEOS (total carbon content in the composite: 7.7%) was sonicated using an ultrasonic probe for 5 min. Subsequently, the gelation process was allowed to take place in an ultrasonic bath for 12 h.13 For aging, the resulting composite gel was immersed in 10−2 m NaOH for 96 h and dried at room temperature. The composite-gel was then milled using a mortar and a pestle, and compressed uniaxially at 500 MPa. Finally, the pellet was sintered at 1150 °C for 15 h in 300 mbar Ar atmosphere. Transmission electron microscopy (TEM) specimens were prepared from the bulk composite by dimpling and ion-milling (Gatan PIPS Model 591 at 3,8 kV).
TEM was performed using a JEOL 2000 FX operating at 200 kV, HRTEM in a JEOL 4000 EX operating at 400 kV, and EELS using a GATAN DigiPEELS 766 on a dedicated STEM VG HB 501UX operating at 100 kV. Line scans were recorded by rastering the electron beam in a line perpendicular to the tube axis in 2 nm steps, measuring the EEL-spectrum for each step.
Fig. 1 (top view) shows a TEM image of a typical coated MWNT found in the composite. Elemental distribution profiles derived from the EELS line scan analysis across this structure is shown in Fig. 1 (bottom view). Silicon and oxygen profiles match and show maxima on both sides of the tubular structure, corresponding to an SiO2 coating. The carbon tube intensity dominates the centre of the profile (dashed line). The coating thickness derived from this intensity profile is ca. 20–30 nm.
 | ||
| Fig. 1 A typical MWNT coated with SiO2 sticking out from the CNT/SiO2-bulk composite (top image). An EELS line scan analysis of the structure from for the Si-L, C-K and O-K edges, indicates that the tubular composite consists of a CNT wrapped by a SiO2-coating of 20–30 nm thickness (bottom image). | ||
An HRTEM image of a cracked SiOx coated tube (only observed once in our studies) is depicted in Fig 2a. The broken segments are shifted apart by ca. 40 nm and held together by the tube. A magnification of the cracked region (Fig. 2b) reveals an undamaged inner MWNT consisting of 15 concentric carbon cylinders. However, higher magnification of the cracked region at the interface of the tube and the coating (Fig. 2b) reveals heavily damaged outer graphene layers (white arrow). This suggests that the outer nanotube shells (strongly bonded to the SiOx matrix) broke apart along the fault of the SiOx-coating, and slipped off together with the coating. Therefore, we believe that there is a strong bond in the C/SiOx-interface due to the carbothermal reduction. Sliding took place between the unreacted inner MWNT and the strongly bonded C-SiOx shell(s) (black arrow in Fig 2b).
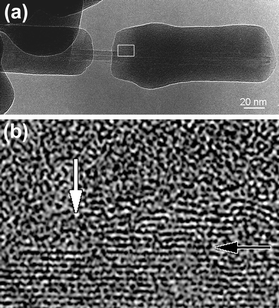 | ||
| Fig. 2 (a) SiOx-coated tube produced at 1150 °C, which is broken (unusual in our samples). The inner cylinder is composed of a MWNT with 15 undamaged cylinders; (b) magnification of the crack region (as marked in a) exhibits broken graphene sheets on the surface of the MWNT (white arrow). The black arrow indicates the proposed glide plane between two cylinders of the tube. | ||
Thermogravimetric (TGA) studies (see ESI†) demonstrate that the coated nanotubes are extremely resistant to oxidation, remaining intact after heating in air up to 1200 °C, provided the carbon tubes are completely embedded in the SiO2 matrix.
In order to understand various interactions and the stability of idealized SiOx coated tubes, we carried out a series of DFTB calculations on single-walled carbon nanotubes (SWNTs) covered by an individual SiOx layer. The DFTB method has been described elsewhere11 and its applicability to SiC and SiO systems has also been demonstrated.14
We studied theoretically the coating of a (20,20) carbon tubule coated with a SiO4 tetrahedral cylinder (Fig 3). Various systems involving the formation of different SiOx (x = 3/2, 2, 5/2) coatings were created. These include: (i) Si–C interactions; (ii) Si–O–C bonds, and (iii) non-bonding interactions between the carbon tube and the oxide layer.‡
 | ||
| Fig. 3 Molecular model of (20,20) carbon tubules covered by: (a) a cylindrical SiOx layer (x = 5/2); (b) a tubular SiOxlayer (x = 3/2). The O atoms are drawn in black. Note the six-membered SiO4 rings on side views. | ||
The structure shown in Fig. 3a represents the relaxed arrangement for a stable C/SiOx (x = 5/2) composite nanotube containing covalent bonds at the interface. This decoration exhibits six-membered SiO4 rings within the SiOx layer (Fig. 3a). However, Si–C bonds appear to be the only stable intertubular connections. It is noteworthy that bonding using Si–O–C bridges does not lead to a stable structure within the SiOx layer (when x = 2) and the carbon nanotube (not shown here).
In addition, we also considered a (20,20) carbon nanotube coated with a SiOx monolayer (x = 5/2), without bonding interaction between the SiOx layer and the carbon tube. Interestingly, an individual SiOx cylinder without a carbon nanotube in the core is unstable. The latter tends to collapse because the low average coordination number (K < 3) is obviously responsible for an insufficient stiffness of the tubular structure.
The formation of (Si–C) bonds between the inner and outer tube (Fig. 3a) leads to a partial rehybridization of the carbon atoms (sp2 → sp3). The decoration of this specific SiOx layer does not lead to any bonding frustration of carbon atoms (e.g. no dangling bonds appear). In this case, localized benzene-like 6π-electron systems are separated by the sp3 carbon atoms, contrary to a delocalized graphene-like π-electron system.
It is important to note that such decorations with SiC bonds, shown in Fig 3, would be unstable for narrow carbon nanotubes, which exhibit larger curvature. For example stable decoration for a (10,10) carbon nanotube was not observed.
The composites were fabricated using novel sol–gel techniques in conjunction with pressure methods and thermal annealing. These materials have proved to be oxidation resistant at temperatures <1200 °C. The results widen the horizons of MWNT/matrix interactions, vital for producing MWNT-reinforced composites (see ESI†). For uniform SiOx coatings, we have proposed that ideally SiOx can be deposited on MWNTs: (i) by establishing covalent bonds between Si and C, or (ii) without links between the SiOx shell and the MWNT outer shell. In this context, we point out that our DFTB models represent a perfect SiOx monolayer interacting with the tube surface. However, we envisage the possibility of achieving such coatings if experimental conditions and SiOx deposition can be fully controlled. It is believed that the optical and electronic properties of these composite tubes are intriguing and a full discussion will be given elsewhere. Finally, coated carbon nanotubes with monolayers of different materials will open up a new area of nanotechnology research and the fabrication of ‘smart’ composite materials.
We thank CONACYT-México grant-W8001 Millennium Initiative (MT), the German Israeli Foundation (TK), the RS (NG), EU grants: NANOCOMP HPRN-CT-2000-00037 (MR, TS), CNT-NET G5RT-CT2001-05026 (MT) and DFG grant Ru342/11-2 (MR, TS) for financial support.
Notes and references
- M. Terrones, W. K. Hsu, H. W. Kroto and D. R. M. Walton, Nanotubes: A Revolution in Material Science and Electronics, in Fullerenes and Related Structures, Topics in Chemistry Series, ed. A. Hirsch, Springer-Verlag, Berlin, 1988, vol. 199, ch. 6, pp.189–234 Search PubMed.
- T. Kuzumaki, O. Ujiie, H. Ichinose and K. Ito, Adv. Eng. Mater., 2000, 2, 416 CrossRef CAS.
- I. Musa, M. Baxendale, G. A. J. Amaratunga and W. Eccleston, Synth. Met., 1999, 102, 1250 CrossRef CAS.
- K. Yoshino, H. Kajii, H. Araki, T. Sonoda, H. Take and S. Lee, Fullerene Sci. Technol, 1999, 7, 695 CAS.
- M. J. Treacy, T. W. Ebbesen and J. M. Gibson, Nature, 1996, 381, 678 CrossRef CAS.
- E. W. Wong, P. E. Sheehan and C. M. Lieber, Science, 1997, 277, 1971 CrossRef CAS.
- P. Calvert, Nature, 1999, 399, 210 CrossRef CAS.
- T. Seeger, Ph. Redlich, N. Grobert, M. Terrones, D. R. M. Walton, H. W. Kroto and M. Rühle, Chem. Phys. Lett., 2001, 339, 41 CrossRef CAS.
- B. C. Satishkumar, A. Govindaraj, M. Nath and C. N. R. Rao, J. Mater. Chem., 2000, 10, 2115 RSC.
- B. C. Satishkumar, A. Govindaraj, E. M. Vogl, L. Basumallick and C. N. R. Rao, J. Mater. Res., 1997, 12, 604 Search PubMed.
- D. Porezag, Th. Frauenheim, Th. Köhler, G. Seifert and R. Kaschner, Phys. Rev. B, 1995, 51, 12947 CrossRef CAS.
- T. W. Ebbesen and P. M. Ajayan, Nature, 1992, 358, 220 CrossRef CAS.
- M. Tarasevich, Am. Ceram. Bull., 1985, 63, 500 Search PubMed.
- R. Gutierrez, Th. Frauenheim, Th. Köhler and G. Seifert, J. Mater. Chem., 1996, 6, 1657 RSC.
- R. Kaschner, Th. Frauenheim, Th. Köhler and G. Seifert, J. Comput.-Aided Mater Des., 1997, 4, 53 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: TGA studies, SEM image of an MWNT/SiOx composite after TEM measurement, and mechanical properties. See http://www.rsc.org/suppdata/cc/b1/b109441f/ |
| ‡ The ‘initial-guess’ tubules have been fully relaxed with respect to the atomic positions using conjugate gradient techniques. Dangling bonds on the oxygen atoms were saturated with hydrogen, and periodic boundary conditions were applied along the tube direction. |
| This journal is © The Royal Society of Chemistry 2002 |
