A non-aqueous organometallic route to highly monodispersed copper nanoparticles using [Cu(OCH(Me)CH2NMe2)2]
Julia
Hambrock
,
Ralf
Becker
,
Alexander
Birkner
,
Jurij
Weiß
and
Roland A.
Fischer
*
Lehrstuhl für Anorganische Chemie II, Organometallics & Materials Chemistry, Ruhr-Universität Bochum, Universitätsstraße 150, 44780, Bochum, Germany. E-mail: rfischer@aci.ruhr-uni-bochum.de
First published on 21st December 2001
Abstract
Good quality, highly monodispersed capped copper metal nanoparticles have been synthesised in a non-hydrolytic approach using thermal decomposition of the Cu(II) precursor [Cu(OCH(Me)CH2NMe2)2] in a hot coordinating solvent without further reducing agents; the copper nanoparticles have been characterised by optical spectroscopy (UV/VIS), electron microscopy (TEM), electron diffraction (SAED), and dynamic light scattering (DLS).
The study of nanoparticles of metals has been an extremely active area of research in recent years because of their unusual properties which are different from those of bulk materials. Since heterogeneous catalysis is a surface phenomenon and the surface to volume ratio increases with decreasing particle diameter, nanoparticles are vital for high catalytic activity. Copper particles, for example, are known to enhance the catalytic activity and selectivity of ZnO in hydration and dehydration reactions e.g. methanol synthesis, and play an important role in solid-oxide fuel cells.1 To prevent nano-sized particles from aggregation capping ligands or other support materials (such as metaloxides) have to anchor to the surface, i.e. at least partially cover the particle. Capped nanoparticles can be obtained in many ways using reducing agents,2 reverse micelles,3via photoreduction,4 γ-irradiation,5 sonochemical6 or radiolytic7 methods. However, there is a lack of methods that allow non-aqueous media which at the same time are chemically simple systems and require few starting materials, no reducing agents or special additives (i.e. detergents). Ideally, no salts or other by-products that may be difficult to remove should be produced. Furthermore, possibilities for systematic optimisation (precursor) as well as controlling (size and surface of the resulting nanoparticles) should be available. There is one area that has accumulated a high amount of consolidated knowledge on precursor chemistry in order to deposit thin metal films: CVD (chemical vapour deposition). Since colloids, on the other hand, can be considered as ‘soluble surfaces’ it appears reasonable to transfer the conceptions of CVD precursor development to solution-based approaches to metal colloids. Moreover, a well controllable reaction system made up of only a coordinating solvent (initially developed for II–VI semiconductor nanoparticles8) can be used for the synthesis of nanoparticles via pyrolysis of a molecularly designed and tuned precursor. This approach takes not only advantage of the concepts of CVD, but offers the control over both parameters that control the features of nanoparticles, the starting material and the reaction system.
Here, we present a relatively simple, reproducible, and non-hydrolytic approach to copper nanoparticles using a known CVD precursor9 [Cu(OCH(Me)CH2NMe2)2] 1 in a pyrolysis procedure in hot coordinating solvents (hexadecylamine, tri-n-octylphosphine oxide). No additional reducing agents are required for this transformation which yields highly mono-dispersed nanoparticles of metallic copper and 1-dimethylaminopropan-2-ol and 1-dimethylaminopropan-2-one as volatile by-products. We can fine-tune the synthesis procedure by varying the parameters of the reaction procedure, e.g. temperature, concentration, reaction time, or solvent, and thereby we achieve the desired control over the central features of the resulting nanoparticles.
The precursor [Cu(OCH(Me)CH2NMe2)2] 1 was prepared as described by Buhro and coworkers,10 by reacting copper(II) methanolate with the corresponding aminoalcohol in an alcohol-exchange procedure. In the following, a typical synthesis is described yielding highly monodisperse copper nanoparticles with 7.5 nm particle diameters. 7 g of hexadecylamine was heated to 100 °C for 1 h under Ar that was repeatedly evacuated to remove oxygen and water. A solution of 0.3 M 1 in octylamine was treated the same way at 60 °C. The reaction was initiated by the rapid injection of 4 mL copper stock solution into the hexadecylamine at 300 °C under vigorous magnetic stirring and an Ar atmosphere. Upon injection of the dark brown precursor solution the colourless amine solution turned red. After the solution was heated for 30 min at 225 °C, the reaction was stopped by cooling to room temperature. Adding toluene to the solid reaction mixture yielded a clear, deep-red dispersion of copper nanoparticles which was very air sensitive as indicated by a colour change of the solution from red to blue. Further purification was achieved by addition of methanol giving a clear colourless solution and a red precipitate. The precipitate was separated by centrifugation carried out in an argon-fluted drybox (H2O, O2 <1 ppm), washed repeatedly with methanol to remove excess solvent, and redispersed in toluene. The resulting colloids are stable for months at room temperature in the absence of air.
The UV/VIS spectrum of the 7.5 nm copper nanoparticles in toluene [Fig. 1(a)] displays a sharp absorption peak at 566 nm. The absorption peak can be attributed to the excitation of plasmon resonance or interband transitions excitation and are characteristic properties of the metallic nature of the particles.11 A TEM image [Fig. 1(b)] confirms the formation of nanoscale copper material as well defined, spherical particles with a diameter of about 7.5 nm. The size and shape of the nanocrystals are very uniform and the individual particles are separated by about 2 nm due to their shells of surfactant from neighbouring particles.
 | ||
| Fig. 1 (a) UV/VIS absorption spectrum of copper nanoparticles in toluene synthesised by thermolysis of 1 in hot hexadecylamine. (b) TEM image of individual copper metal nanoparticles showing a tendency towards 2-D ordering. The nanocrystals were deposited from a toluene dispersion onto a carbon support film on a Au-grid: bar = 110 nm. | ||
Obviously, the small size distribution leads to the formation of hexagonally 2-D ordered lattices of free standing copper colloids. However, the nanocrystals appear to be relatively unstable when imaged at higher magnifications and we have been unable as yet to observe lattice planes. The corresponding selected area electron diffraction (SAED) pattern is shown in Fig. 2. Four of the six rings match with the lattice planes (111), (200), (220) and (311), respectively, of the cubic copper phase. However, two additional rings in Fig. 2(a) of lower intensity and of more diffuse nature are observed in the electron diffraction pattern. The corresponding lattice distances can be attributed to both the lattice planes (111) and (220) of copper(I) oxide and to the (−111) and (−113) planes of copper(II) oxide. These reflections are also observed for larger particles but in this case they are even less intense in accordance with the decreasing surface to volume ratio. However, when the TEM grid was prepared and transported in an inert gas atmosphere using a vacuum transfer holder, reflections stemming from copper oxides were no longer observed [Fig. 2(b)]. From these findings it can be concluded that the produced particles consist of pure metallic copper whose surface may oxidise upon contact with air yielding weak reflection rings in addition to intense and more definite reflections stemming from the unaffected metal cores. An EDX (energy-dispersive X-ray) analysis performed on the nanoparticles results in a spectrum with peaks for copper (with additional peaks from the coordinating solvent and the support grid respectively) independently confirming the composition of the sample.
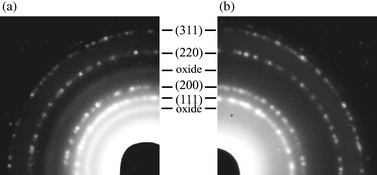 | ||
| Fig. 2 Electron diffraction pattern of the copper nanoparticles shown in Fig. 1(b). (a) Contact with air during transport; (b) prepared and transported in an inert gas atmosphere. | ||
Toluene solutions of the copper nanocrystallites were further subjected to dynamic light scattering (DLS) using a ALV-5000E correlator. DLS provides the mean hydrodynamical radius of particles by fitting the experimentally observed autocorrelation function to a theoretical function that contains the diffusion coefficient D and hence the radius r. The distribution function displays only one major peak centred at r = 5 nm beside some minor contributions from larger particles (the weight of these peaks is only 10−9 to 10−14) confirming the small size distribution of the sample. However, the resulting diameter of the particles (d = 10 nm) is larger than that obtained by TEM (d = 7.5 nm). The difference of 2.5 nm can be attributed to ligands that surround the metallic core of the particles, i.e., hexadecylamine, preventing them from aggregation and providing an additional solvent shell. This corresponds very well to the distance between two particles observed in TEM.
It has been reported that the size and shape of copper colloids can be controlled by various additives.12 Furthermore, the reaction system applied is known to influence the shape of semiconductor nanoparticles.13 Therefore, the reaction was carried out under the same conditions using other solvents, stabilising agents and additives such as tri-n-octylphosphine oxide (TOPO). In TOPO alone, we found that a dark precipitate was formed which was not soluble in toluene. The corresponding TEM images [see Fig. 3(a)] reveal that larger, mostly spherical particles of different sizes ranging from 50 nm to 3.7 μm have been formed. Hence, the use of TOPO results in particles that are larger by a factor of at least 10 compared to the reaction in hexadecylamine, explaining the observed insolubility. However, the reaction is not only influenced by the coordinating solvent. If a solution of 0.3 M 1 in tri-n-butylphosphine is injected into hot TOPO, the diameter of the particles decreases and various particle shapes can be seen in the TEM images [see Fig. 3(b)]: spherical, oval and, surprisingly, elongated and cylindrical copper metal particles are observed. Their sizes range from 8 to 60 nm for spherical particles and 15–40 (short axis) and 50–100 nm (long axis) for the elongated particles, respectively. However, elongated particles are observed only for high precursor concentrations. If we use only 0.4 mL stock solution under otherwise constant conditions, rod-shaped particles are not observed.
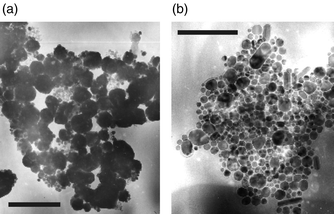 | ||
| Fig. 3 (a) TEM image of insoluble copper nanoparticles synthesised (a) by thermolysis of 1 in hot tri-n-octylphosphine oxide (TOPO): bar = 200 nm. (b) by thermolysis of a solution of 1 (in tri-n-butylphosphine) in hot TOPO: bar = 950 nm. | ||
In conclusion, we have shown that a simple, low cost CVD precursor is particularly suited for the synthesis of copper nanoparticles. The controlled reaction leads to the formation of very uniform particles showing a 2-D ordering if hexadecylamine is used as the coordinating solvent. Because of the simplicity of the method, many opportunities may exist to further control the size and shape of Cu nanoparticles. Furthermore, we suggest as a general principle that other organometallic CVD precursors may be very good choices for the synthesis of nanometals.
This work was supported by the German Research Foundation (SFB 558/B1). Valuable experimental assistence of C. Schirrmacher is gratefully acknowleged.
Notes and references
- S. Park, R. J. Gorte and J. M. Vohs, Appl. Catal. A, 2000, 200, 55 CrossRef CAS.
- S. Ayyappan, R. S. Gopalan, G. N. Subbanna and C. N. R. Rao, J. Mater. Res., 1997, 12, 398 Search PubMed; M. J. Williams and P. P. Edwards, Faraday Discuss. Chem. Soc., 1991, 92, 199 Search PubMed.
- I. Lisiecki and M. P. Pileni, J. Am. Chem. Soc., 1993, 115, 3887 CrossRef CAS.
- H. H. Huang, X. P. Ni, G. H. Loy, C. H. Chew, K. L. Tan, F. C. Loh, J. F. Deng and G. Q. Yu, Langmuir, 1996, 12, 909 CrossRef CAS.
- S. S. Joshi, S. F. Patil, V. Iyer and S. Mahumuni, Nansotruct. Mater., 1998, 10, 1135 Search PubMed.
- K. Okitsu, H. Bandow and Y. Maeda, Chem. Mater., 1996, 8, 315 CrossRef CAS.
- E. Janata, Radiat. Chem., 1996, 47, 29 Search PubMed; T. Sosebee, M. Giersig, A. Holzwarth and P. Mulvaney, Ber. Bunsenges. Phys. Chem., 1995, 99, 40 Search PubMed.
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 7806 CrossRef; J. E. B. Katari, V. L. Colvin and A. P. Alivisatos, J. Phys. Chem., 1994, 98, 4109 CrossRef.
- R. Becker, J. Weiß, A. Devi and R. A. Fischer, J. Phys. IV, 2001, 11, 569 Search PubMed.
- S. C. Goel, K. S. Kramer, M. Y. Chiang and W. E. Buhro, Polyhedron, 1990, 9, 611 CrossRef CAS.
- J. A. Creighton and D. G. Eadon, J. Chem. Soc., Faraday Trans., 1991, 87, 3881 RSC.
- A. Filankembo and M.-P. Pileni, Appl. Surf. Sci., 2000, 164, 260 CrossRef CAS.
- Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2001, 123, 1389 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |
