A specificity-enhanced time-resolved fluoroimmunoassay for zeranol employing the dry reagent all-in-one-well principle
Mika
Tuomola
*a,
Kevin M.
Cooper
b,
Susanne
Lahdenperä
a,
G. Andrew
Baxter
c,
Christopher T.
Elliott
c,
D. Glenn
Kennedy
c and
Timo
Lövgren
a
aDepartment of Biotechnology, University of Turku, Turku, FIN-20520 Finland. E-mail: mika.tuomola@utu.fi; Fax: +358 2 333 8050; Tel: +358 2 333 8089
bDepartment of Veterinary Science, Queen’s University Belfast, Stoney Road, Stormont, Belfast, UK BT4 3SD
cDepartment of Agriculture and Rural Development, Veterinary Sciences Division, Stoney Road, Belfast, UK BT4 3SD
First published on 5th December 2001
Abstract
A simple dry chemistry time-resolved fluorescence immunoassay (TR-FIA) method was developed for the measurement of zeranol in bovine urine samples. The samples were purified by immunoaffinity chromatography and a specificity-enhanced zeranol antibody was employed in the immunoassay. This resulted in a highly selective method, which had only negligible reactivity with Fusarium spp. toxins. The all-in-one-well dry chemistry concept made the assay very simple to use because all the assay-specific reagents were already present in the reaction wells in dry form. Only the addition of diluted sample extract was required to perform the competitive one-step TR-FIA and the results were available in less than 1 h. The analytical limit of detection (mean + 3s) for the immunoassay was 0.16 ng ml−1 (n = 12) and the functional limit of detection for the whole method, estimated by the analysis of zeranol-free samples, was 1.3 ng ml−1 (n = 20). The recovery of zeranol at the level of 2 ng ml−1 was 99% (n = 18) and the within-assay variation ranged between 4.5 and 9.0%.
Introduction
Zeranol is a non-steroidal anabolic agent derived from the naturally occurring mycotoxin zearalenone.1 This semi-synthetic oestrogen improves feed efficiency and increases the rate of weight gain in ruminants. However, its use in food animals has been banned within the EU.2 The ban is monitored in EU Member States by carrying out random tests for the presence of zeranol.3A number of immunochemical methods have been developed for the screening of zeranol.4–7 However, all the current immunochemical tests exhibit cross-reactivity to a greater or lesser extent with zearalenone, α-zearalenol and β-zearalenol, the three principal toxins produced by Fusarium spp. fungi. The structures of these compounds are closely related to that of zeranol (Fig. 1). As a consequence, these screening assays may show positive results in samples containing these toxins, irrespective of whether zeranol itself is actually present or not. In addition, current immunoassays usually require multiple assay steps, which slows the test throughput and makes them more prone to errors. Simpler assay formats are needed to make the methods more reliable and user friendly.
Time-resolved fluorescence immunoassays (TR-FIAs) based on lanthanide chelates are well established in human in vitro diagnostics. This technology is based on the unique fluorescent properties of the chelates as characterised by narrow-band emission lines, long Stokes shift and long fluorescence lifetimes. The long emission duration is utilised in the delayed measurement of specific fluorescence, which is distinct from short-lived background signal.8
Although there are currently no commercially available TR-FIA kits for veterinary drug residue analysis, conventional diagnostic products have been adapted for analysis of steroid hormones in bovine plasma.9 TR-FIA methods have been developed for determination of medroxyprogesterone in bovine bile,10 monensin in poultry plasma11 and ivermectin in bovine milk.12 We have previously demonstrated that TR-FIA may also be used in a dry chemistry all-in-one-well format, which considerably simplifies the assay procedure.13All analyte-specific reagents are pre-dispensed and dried in microtitre wells, requiring only the addition of diluted sample extract at the time of analysis. After a short incubation the wells are washed and the time-resolved fluorescence signal is measured following a signal enhancement step.
Here we report the development of a TR-FIA screening method for zeranol capable of distinguishing between zeranol and the Fusarium spp. toxins. The screening performance of the assay has been improved by inducing tolerance in the antibody against selected cross-reactive compounds. As demonstrated previously,14 unwanted antibody cross-reactivity may be reduced by priming the host with the interfering cross-reactant coupled to a non-immunogenic amino acid copolymer to inactivate the B-lymphocyte clones specific for the cross-reactant, producing a specific immunotolerance. The tolerant host is then immunised with the hapten against which the specific antibody is required. The application of this technique has resulted in an specific antibody for zeranol with negligible cross-reactivity for the Fusarium spp. toxins. The antibody has been used to develop a rapid and user-friendly TR-FIA method employing the dry chemistry principle and the all-in-one-well format.
Experimental
Reagents and chemicals
All chemicals, unless stated otherwise, were supplied by Sigma (St. Louis, MO, USA). Bovine serum albumin (BSA) was supplied by Intergen (Chicago, IL, USA). Microtitration plates coated with goat anti-rabbit IgG, Delfia Wash Solution and Delfia Enhancement Solution were purchased from Perkin-Elmer Life Sciences (Wallac, Turku, Finland).Antibody production
A polyclonal antiserum against zeranol was raised in rabbits using the method of specificity-enhancement described previously.14 Carboxypropyl ether derivatives at C-16 (16-CPE) of zeranol and the Fusarium spp. toxin α-zearalenol were prepared as described by Dixon and Russell.15 The 16-CPE derivative of zeranol was conjugated to the immunogenic carrier protein bovine thyroglobulin (BTG) using the acid anhydride method of Erlanger et al.16 The 16-CPE derivative of α-zearalenol was conjugated to the random copolymer of D-glutamic acid and D-lysine (DGL) by the same method. A rabbit was immunised with the α-zearalenol-DGL tolerogen to inactivate the B-lymphocyte clones specific for this toxin. Three days later the host was immunised with zeranol–BTG immunogen. Booster injections were administered and antiserum was harvested as described previously.14 Another polyclonal antiserum was raised in a goat to be used in the preparation of immunoaffinity columns. Immunisation was carried out every 4 weeks using zeranol–BTG immunogen and the antiserum was harvested after 63 d.Measurement of antibody cross-reactivity
The antibody cross-reactivity with the Fusarium spp. toxins and metabolites of zeranol was estimated as described by McCaughey et al.17 The selectivity of the specificity-enhanced zeranol antibody was determined in a TR-FIA system and the cross-reactivity of the caprine antibody was determined by competitive ELISA using rabbit anti-goat antibody-coated plates (Perkin-Elmer Life Sciences) and a zeranol–16-CPE–horseradish peroxidase conjugate prepared as described above.15,16Preparation of europium labelled hapten–ovalbumin conjugate
The zeranol 16-CPE derivative was activated by using a modified N-hydroxysuccinimide (NHS) active ester method.18 The hapten (44 μmol) was incubated overnight at room temperature with 46 μmol of N-hydroxysuccinimide and dicyclohexylcarbodiimide in 1.5 ml of 1,4-dioxane. The product was evaporated to dryness and NHS-activated zeranol was purified using preparative thin-layer chromatography. The activated hapten and isothiocyanate derivative of the europium-labelling reagent, [2,2′2″2′″-({4-[(4-isothiocyanatophenyl)ethynyl]pyridine-2,6-diyl}bis(methylenenitrilo))tetrakis(acetato)]europium (III) (Eu-W1024-ITC chelate) (Perkin-Elmer Life Sciences), were coupled to ovalbumin (Sigma A-5503) in a single reaction. Typically, 2 mg of ovalbumin were combined with a 2× and 60× molar excess of NHS-activated zeranol and labelling reagent, respectively. The reaction was carried out overnight at room temperature in 0.1 mol l−1 borate buffer (pH 8.6). The Eu-labelled hapten–ovalbumin conjugate was purified by gel filtration on a Superdex 200 HR 10/30 column (Pharmacia Biotech, Uppsala, Sweden) with 50 mmol l−1 TRIS–HCl (pH 7.75), 0.9% NaCl, 0.05% NaN3 as elution buffer.Preparation of dry chemistry microtitre plates
Zeranol antibody was diluted to a suitable working titre with 50 mmol l−1 TRIS–HCl buffer, pH 7.75, containing 0.9% NaCl, 0.5% BSA, 0.05% NaN3, 0.01% Tween 40, 0.05% bovine γ-globulin, 20 μM diethylenetriaminepentaacetic acid and 20 μg ml−1 Cherry Red. A 50 μl volume of the solution was incubated in goat anti-rabbit IgG-coated microtitration wells for 2 h at room temperature and the wells were washed four times with wash solution. The insulating carbohydrate layer was prepared by adding to the wells 50 μl of 50 mmol l−1 phosphate buffer, pH 7.2, containing 4% trehalose, 0.9% NaCl, 0.1% casein, 0.05% NaN3 and 0.01% Brij 35. The solution in the wells was dried overnight at 35 °C. The labelled hapten–ovalbumin conjugate was diluted in insulating layer buffer and 7 ng of label were dispensed on top of the dry insulating layer in a 1 μl volume. The solution was immediately dried by blowing air on to the well. The all-in-one dry wells were stored at room temperature in sealed packages with desiccant.Preparation of immunoaffinity chromatography (IAC) columns
Immunoglobulin G was purified from the goat polyclonal antiserum by precipitation using a combination of caprylic acid (Cat. No. C-2875, Sigma; add 0.25 ml slowly to 5 ml of antiserum and 15 ml of acetate buffer, pH 4.5, stir for 30 min and recover the supernatant) and saturated ammonium sulfate solution (add to supernatant at pH 7.4 to give 45% ammonium sulfate solution, stir for 30 min and recover the precipitated IgG in phosphate-buffered saline, pH 7.4). Purified IgG was coupled at pH 9.0 to UltraLink Biosupport Medium (Cat. No. 53111, Pierce, Rockford, IL, USA) at 12 mg of protein per millilitre of gel and 0.75 ml of gel was packed into 9 mm diameter polypropylene columns (IST, Hengoed, Mid-Glamorgan, UK).Sample preparation
A bovine urine sample (250 μl) was added to 750 μl of phosphate buffer (0.5 mol l−1, pH 7.4) and incubated overnight at room temperature, or for 2 h at 37 °C, with 10 μl of β-glucuronidase from Escherichia coli (Roche Diagnostics, Lewes, East Sussex, UK). Zeranol IAC columns were pre-conditioned with 4 ml each of 0.5 mol l−1 NaCl, 80% v/v ethanol and de-ionised water. Deconjugated sample was applied to the column under gravity. The loaded column was washed with 3 ml of 0.5 mol l−1 NaCl, 3 ml of 0.25 mol l−1 NaCl and 9 ml of de-ionised water. The column was primed by application of 0.25 ml of 80% ethanol. Sample was eluted and collected by applying 2.25 ml of 80% ethanol. Columns were reconditioned with 4 ml of 80% ethanol and 4 ml of de-ionised water and stored at 4 °C in 20% ethanol. The sample eluate was evaporated to dryness at 85 °C under nitrogen and reconstituted in 0.5 ml of de-ionised water heated to ∼50 °C to aid solvation. The sample could then be applied to zeranol TR-FIA plates.Dry chemistry TR-FIA protocol
Sample extracts and zeranol standards prepared in de-ionised water were applied to plates in triplicate 50 μl aliquots. The plates were shaken for 15 min at room temperature before being washed four times with wash buffer. Enhancement solution (200 μl) was added to each well and the plates were shaken for 20 min at room temperature. Fluorescence was then measured with a Victor 1420 multi-label counter with on-line data reduction MultiCalc software, supplied by Perkin-Elmer Life Sciences.TR-FIA validation
Assay validation was performed using Community Reference Laboratory Bank of Reference Blank Bovine Urines kindly supplied by RIVM (Bilthoven, The Netherlands).Results and discussion
The use of tolerance induction in the antibody production resulted in a highly specific zeranol antiserum. As summarised in Table 1, the specificity-enhanced antibody exhibited negligible cross-reactivity against fungal metabolites. The absence of reactivity against α-zearalenol is of particular importance because antibodies used in past methods have always shown significant cross-reactivity against this compound, typically ranging from 21 to 54%.6,7,15,19,20 With the exception of taleranol, the reactivity profile was slightly better than that obtained in an ELISA system also employing the specificity-enhanced zeranol antibody.14 The cross-reactivity against taleranol was three times higher in the current study but still in the range found in some other zeranol antibodies.6,19 This should not be a problem for a screening assay because taleranol levels have been found only just to reach the corresponding zeranol concentrations in real samples. On the other hand, the zearalenols/zeranol ratio has been shown to exceed 10∶1.20–22 In addition, unlike both α-and β-zearalenol, taleranol is a metabolite of zeranol and its presence may also be indicative of drug abuse.The label components used in competitive TR-FIA methods can be synthesised either by attaching the lanthanide chelate directly to the hapten23 or by coupling both the hapten and the chelate label to a carrier molecule, generally a protein.10,11The latter option is useful for increasing the labelling degree and thus the signal level of the label. However, the conjugation reactions for haptens and lanthanide chelates are usually done sequentially, which complicates the label preparation process and makes the control of coupling ratios (haptens/lanthanide chelates per carrier molecule) difficult. The label synthesis scheme was simplified in the current study by carrying out both the hapten and lanthanide chelate attachment in a single reaction. The results were highly reproducible because the hapten density and labelling degree of the carrier molecule could be adjusted simply by changing the initial molar ratios of reagents.
The use of the dry chemistry concept made the assay very simple for the end-user because all the assay-specific components were already present in the wells and the assay was started by adding only the diluted sample to the well. The dry layer in the wells not only acted as insulating material between the primary antibody and zeranol label but also contained all the assay buffer components which became solubilised when the sample was added. The influence of several additives such as carbohydrates, proteins and detergents was tested with both these goals in mind. In agreement with our previous results,13 a dry layer consisting of both protein and carbohydrate, in this case casein and trehalose, produced the best results. Several detergents were also tested in order to decrease the non-specific binding of assay components. A significant improvement (Fig. 2) was reached in both the assay sensitivity and repeatability when detergents were used at their optimal level (0.01%).
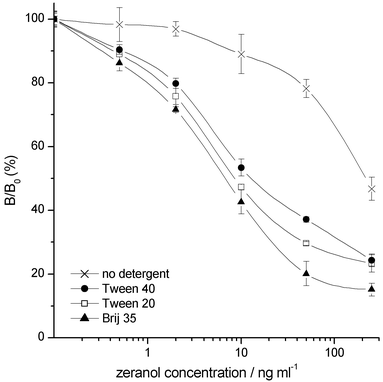 | ||
| Fig. 2 Effect of using different detergents in the dry reagent layer on the zeranol TR-FIA repeatability and sensitivity. | ||
Fig. 3 shows a typical zeranol dry chemistry TR-FIA calibration curve. The mean mid-point of the calibration curve (n = 15) was 1.11 ng ml−1 (SEM 0.05 ng ml−1). The use of surface measurement was briefly tested because the labels contained stable and inherently fluorescent europium chelates, which allowed the direct measurement of fluorescence without any enhancement step. Instead of adding enhancement solution, the microwells were dried rapidly under an air stream after the washing step. The fluorescence signal obtained by a surface measurement mode was ∼50% lower than the respective signal in assays using enhancement solution. The response curve was similar to that obtained in liquid measurements and the repeatability was good (RSD <5%) in the hands of experienced users. The fluorescence signal of the present lanthanide chelate was, however, susceptible to quenching by ambient humidity, which made the results too operator-dependent and the use of an enhancement step was considered necessary in the manual assay system.
 | ||
| Fig. 3 Zeranol calibration curve for the final dry chemistry TR-FIA method (n = 6). | ||
The analytical limit of detection for the zeranol TR-FIA, based on the mean plus three standard deviations of the measured concentrations of the zero calibrator (n = 12), was calculated as 0.16 ng ml−1. The functional limit of detection for the whole method was determined by analysing bovine urine samples known to be free from zeranol (n = 20) on three days using three different batches of IAC column. The results ranged from 0 to 1.1 ng ml−1 with a mean value of 0.5 ± 0.3 ng ml−1 (n = 59) and the limit of detection (mean + 3s) was calculated as 1.32 ng ml−1. The accuracy and repeatability of the method were determined by repeating the analysis of six blank bovine urines fortified with 2 ng ml−1 of zeranol over three days (Table 2). The average recovery of zeranol was 99% and the intra-assay variation ranged from 4.5 to 9.0%.
| Day 1 | Day 2 | Day 3 | Overall | |
|---|---|---|---|---|
| Mean value/ng ml−1 | 2.11 | 1.74 | 2.08 | 1.98 |
| s/ng ml−1 | 0.09 | 0.16 | 0.16 | 0.22 |
| RSD (%) | 4.5 | 9.0 | 7.7 | 10.9 |
| n | 6 | 6 | 6 | 18 |
In conclusion, we have described a novel method for the screening of zeranol residues in bovine urine. Samples were purified by immunoaffinity chromatography directly after deconjugation, which simplified the sample pre-treatment and minimised the use of organic solvents. The immunoassay had high selectivity towards zeranol owing to the use of a specificity-enhanced antibody, which decreases the rate of false-positive results caused by the presence of Fusarium spp. toxins. The all-in-one-well dry reagent immunoassay concept permitted rapid one-step analysis, which was extremely simple for the end-user because the biospecific reaction was started simply by adding a diluted sample extract to the well. The same concept may be readily applied to other analytes in the residue field.
Acknowledgements
The authors acknowledge the financial support of the European Commission for the project FAIR5-CT1997-3443 ‘Natural Zeranol’. We thank the European Union Community Reference Laboratory at the National Institute of Public Health and Environment, Bilthoven, The Netherlands for supplying reference blank urines.References
- P. H. Hidy, R. S. Beldwin, R. L. Greasham, C. L. Keith and J. R. McMullin, Adv. Appl. Microbiol., 1977, 22, 59 Search PubMed.
- Off. J. Eur. Communities, 1996, L125, 3 Search PubMed.
- Off. J. Eur. Communities, 1996, L125, 10 Search PubMed.
- S. N. Dixon, J. Vet. Pharmacol. Ther., 1980, 3, 177 CAS.
- A. P. Carter, S. N. Dixon and M. H. Bew, J. Vet. Pharmacol. Ther., 1984, 7, 17 CAS.
- E. H. J. M. Jansen, R. H. van den Berg, G. Zomer, C. Enkelaar-Willemsen and R. W. Stephany, J. Vet. Pharmacol. Ther., 1986, 9, 101 CAS.
- P. J. Patel and S. N. Dixon, Food Addit. Contam., 1989, 6, 91 CAS.
- E. Soini and T. Lövgren, CRC Crit. Rev. Anal. Chem., 1987, 18, 105 Search PubMed.
- C. T. Elliott, K. S. Francis, H. D. Shortt and W. J. McCaughey, Analyst, 1995, 120, 1827 RSC.
- C. T. Elliott, K. S. Francis and W. J. McCaughey, Analyst, 1994, 119, 2565 RSC.
- S. R. H. Crooks, T. L. Fodey, G. R. Gilmore and C. T. Elliott, Analyst, 1998, 123, 2493 RSC.
- S. R. H. Crooks, P. Ross, C. S. Thompson, S. A. Haggan and C. T. Elliott, Luminescence, 2000, 15, 371 CrossRef CAS.
- T. Lövgren, L. Meriö, K. Mitrunen, M.-L. Mäkinen, M. Mäkelä, K. Blomberg, T. Palenius and K. Pettersson, Clin. Chem., 1996, 24, 1196 Search PubMed.
- G. A. Baxter, C. T. Elliott, S. R. H. Crooks and W. J. McCaughey, Food Agric. Immunol., 1996, 8, 85.
- S. N. Dixon and K. L. Russell, J. Vet. Pharmacol. Ther., 1983, 6, 173 CAS.
- B. F. Erlanger, F. Borek, S. M. Beiser and S. Lieberman, J. Biol. Chem., 1957, 228, 713 CAS.
- W. J. McCaughey, C. T. Elliott and S. R. H. Crooks, Food Addit. Contam., 1990, 7, 259 CAS.
- J. J. Langone and H. Van Vunakis, Methods Enzymol., 1982, 84, 628 Search PubMed.
- J. P. Duchatel and G. Maghuin-Rogister, Ann. Rech. Vet., 1985, 16, 93 Search PubMed.
- D. G. Kennedy, S. A. Hewitt, J. D. G. McEvoy, J. W. Currie, A. Cannavan, W. J. Blanchflower and C. T. Elliott, Food Addit. Contam., 1998, 15, 393 CAS.
- J. E. Roybar, R. K. Munns, W. J. Morris, J. A. Hurlbut and W. Shimoda, J. Assoc. Off. Anal. Chem., 1988, 71, 263 Search PubMed.
- A. F. Erasmuson, B. G. Scahill and D. M. West, J. Agric. Food Chem., 1994, 42, 2721 CrossRef CAS.
- M. Tuomola, R. Harpio, P. Knuuttila, H. Mikola and T. Lövgren, J. Agric. Food Chem., 1997, 45, 3529 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2002 |

