DOI:
10.1039/B106346B
(Paper)
Analyst, 2002,
127, 119-124
A selective PVC membrane for di- or trinitrophenol based on N,N-dibenzyl-3,3′,5,5′-tetramethylbenzidine
Received 17th July 2001, Accepted 1st November 2001
First published on 10th December 2001
Abstract
A new fluorophore, N,N-dibenzyl-3,3′,5,5′-tetramethylbenzidine (NBTMB), was prepared and shown to exhibit significant and analytical usefulness for optical sensing toward 2,4-dinitrophenol or 2,4,6-trinitrophenol (picric acid) when it was immobilized in a plasticized poly(vinyl chloride) (PVC) membrane. When the membrane was applied to aqueous nitrophenol solution, NBTMB was able to extract selectively nitrophenol into the membrane phase. Since the extraction equilibrium was accompanied by fluorescence quenching of NBTMB, the chemical recognition process could be directly translated into an optical signal. The sensor showed reversible response in the concentration range from 2.0 × 10−7 to 6.0 × 10−5 mol L−1 for the detection of 2,4-dinitrophenol in NaOAc–HOAc buffer at pH 4.0. It also showed a fast response time (t95% < 1.5 min) when the sensor was applied
to 2,4-dinitrophenol solution at concentration levels of 5.26 × 10−6 and 2.10 × 10−5 mol L−1 alternatively. A working principle is proposed and the responses of this sensor to various kinds of nitrophenol were studied. The sensor was applied to the direct determination of 2,4-dinitrophenol in prepared water samples and the indirect assay of the drug cinchonine and the results obtained were satisfactory.
Introduction
There is an ever-increasing need for constructing chemical sensors to monitor all aspects of industrial and environmental samples in real time. Nitrophenols are a class of organic compounds widely used in industry and they are serious pollutants in the environment. The monitoring of nitrophenols is essential for environmental pollution control and industrial applications. Conventional methods for the assay of nitrophenols are discontinuous, usually involving classical chemical methods,1 UV–visible spectrophotometry2 or polarogaphy3 after the analytes have beeen reduced and transformed into azo compounds. Methods based on the use of gas chromatography4 and HPLC5–7 have been developed for the direct determination of nitrophenols, but questions remain about how to realize on-line destructive devices. Potentiometry
with ion-selective electrodes8,9 for picric acid (2,4,6-trinitrophenol) has been applied and shows considerbale advantages over other methods in the area of pharmaceutical analysis10 and biological chemical determination.11 Unfortunately, since picric acid ion-selective electrodes are generally based on classical ion exchange, their inherent selectivity pattern may cause interference problems from lipophilic anions.In recent years, optical chemical sensors (optodes) have become a rapidly expanding area of analytical chemistry, because they offer the advantages of simple preparation, reasonable selectivity and sensitivity. The development of optical methods for the detection and determination of environmentally important species is an important area of contemporary sensor research.12,13 Different kinds of optical chemical sensors have been reported in the literature for measuring various environmental species such as ammonia,14 aliphatic amines,15 sulfur dioxide,16 nitrogen dioxide,17 organochlorine compounds,18 nitro compounds,19 surfactants20 and heavy metal ions.21,22 Optical chemical sensors for nitrophenols
were first proposed by Jian and Seitz23 and developed by our group,24,25 in which the susceptible molecules were incorporated into stable polymeric supports by physical entrapment. These sensors, unfortunately, are related to the use of carcinogenic compounds (pyrene or pyrenebutyric acid). Recently, a non-carcinogenic-based fluorescence membrane for picric acid was reported by covalently immobilizing a fluorescein derivative on an optode glass surface.26 Such methods, however, cannot be used as a general approach, since the immobilization usually changes the optical characteristics of the sensing material and the preparation process is cumbersome.
The major challenge for the application of optical chemical sensors to nitrophenols is the search for photochemical sensing materials which fulfil the requirements of recognition of the analytes and expression of a relationship to the optical signals. In a previous study, we found that in aqueous solution, the fluorescence of 3,3′,5,5′-tetramethylbenzidine (TMB) was quenched by nitrophenol and it was developed as a renewable drop sensor for 2,4-dinitrophenol.27 In this paper, we report the first use of a newly synthesized TMB derivative dye, N,N-dibenzyl-3,3′,5,5′-tetramethylbenzidine (NBTMB), for reversible optical sensing of nitrophenols. The optical measuring is based on the reversible fluorescence quenching of the material which was immobilized in a plasticized poly(vinyl chloride) (PVC) membrane. The sensor can respond selectively, rapidly and reversibly to 2,4-dinitrophenol or picric acid.
Experimental
Materials and apparatus
The selected sensing material, NBTMB, was prepared by the reaction of benzaldehyde and 3,3′,5,5′-tetramethylbenzidine (EtOH, room temperature, 24 h), following by reduction in situ of the resulting mixture with NaBH4 according to a reported procedure,28 and the product was verified by IR and 1H NMR spectroscopy. For membrane preparation, high molecular weight PVC, bis(2-ethylhexyl) sebacate (DOS) and tetrahydrofuran (THF) were used. Stock standard solutions of 1.0 × 10−3 mol L−1 nitrophenols were prepared by dissolving the appropriate amount of the nitrophenol in distilled water. Nitrophenols solutions of lower concentration were obtained by serial dilution of the stock standard solution in NaOAc–HOAc buffer of pH 4.0. Except where specified otherwise, all solutions were prepared with distilled water, all other chemicals were of analytical-reagent grade.Preparation of sensing membrane
The sensing membrane M2 (see Table 1) was prepared by using a spin-coating technique. A membrane cocktail was obtained by dissolving 3.0 mg of NBTMB, 50 mg of PVC and 100 mg of DOS in 2.0 mL of freshly distilled THF. An aliquot of 0.2 mL of this solution was applied to the surface of a circular 35 mm diameter quartz plate which was mounted on a rotating (rotating frequency 600 rpm) aluminum alloy rod under a THF-saturated atmosphere. After a spinning time of only 5 s, a membrane of 2–4 μm thickness was obtained on the quartz plate. For comparative studies, in a similar way, membranes containing different amounts of the sensing material were prepared from the described membrane cocktail.
Table 1 Composition of sensing membrane solutiona
| Entry | NBTMB/mg | Linear range/mol L−1 | Response slope/L mol−1 | Correlation coefficient |
|---|
| |
|---|
| Each membrane contains 50 mg of PVC and 100 mg of DOS. Compositions of a given amount were taken up with a microsyringe, mixed with 50 mg of PVC and 100 mg of DOS, then diluted to 2 mL with freshly distilled THF. |
|---|
| M1 | 6.0 | 2.10 × 10−7–2.10×10−5 | 3.55 × 104 | 0.9945 |
| M2 | 3.0 | 2.00 × 10−7–6.00×10−5 | 5.00 × 104 | 0.9967 |
| M3 | 1.5 | 1.00 × 10−5–2.10×10−4 | 5.19 × 104 | 0.9924 |
| M4 | 0.75 | 2.00 × 10−5–2.00×10−4 | 8.10 × 104 | 0.9770 |
Experimental procedure
An identical membrane cast on the quartz plate and a 35 mm diameter black PVC plate were mounted in the special flow-through measuring cell described eleswhere.29 The cell was introduced into a Hitachi spectrophotometer (Model F-2500) at an appropriate position30 to guarantee the detection of the intensity of the fluorescence emission without interference from the excitation source. About 3.4 mL of standard or sample solution were introduced and the fluorescence emission spectra of the optode membrane were recorded in the range 350–630 nm with an excitation wavelength of 328 nm. Before each measurement, all sensing membranes were conditioned in NaOAc–HOAc plain buffer solution until the fluorescence intensity was stabilized. The limiting fluorescence intensities F0 and F1 were determined with the sensing membrane in contact with plain buffer solution and 8.0 × 10−4
mol L−1 of 2,4-dinitrophenol solution, respectively. The theoretical functions were fitted to experimental data for each nitrophenol by performing a non-linear least-squares regression.For the selectivity measurements, the separate solution method (SSM)31was used throughout by using solutions of corresponding nitrophenols in NaOAc–HOAc buffer solution. The complexation constants were calculated based on the experimental data and eqn. (2) (see below).
Results and discussion
NBTMB as a new lipophilic fluorophore
TMB is a non-carcinogenic compound scarcely known as an analytical reagent outside the field of clinical chemistry. It has replaced benzidine (carcinogenic) for the measurement of hemoglobin and has been extensively applied to the spectrophotometric determination of lipid hydroxyperoxides,32 cyanide ion in blood,33 heavy metal ions in water34,35 and chlorine in water36 and air.37 The rigid conjugated backbone and extended unsaturated ring system of the TMB molecule result in a very high quantum yield. However, its water solubility precludes its use in optode membrane preparation. At the onset of this investigation, we designed and synthesized a lipophilic TMB molecule by introducing two benzyl groups into the molecular backbone (Fig. 1), which satisfies the basic condition for a compound acting
as a sensing membrane component.38 The compound in its solid form is extremely stable and can be stored in the dark for a long period of time without any deterioration. After immobilization in a plasticized PVC membrane, the spectral properties of NBTMB in the membrane remain the same as those measured in an organic solvent. |
| | Fig. 1 Structure of NBTMB. | |
Fluorescence response of NBTMB to nitrophenol
NBTMB is a much better fluorescent compound than TMB. The extended unsaturated ring system of NBTMB can emit strong fluorescence in the UV region, which may be related to the resonance interactions of different positions of the molecule and the formation of a quinoid structure with a planar configuration in the excited state:Hence it is possible to form a non-fluorescent ground-state complex by collisional deactivation with compounds containing hydroxyl or nitro groups.39 This ground-state complex may have a very low fluorescence efficiency and account for the decrease in fluorescence intensity emitted. Fig. 2 shows the fluorescence emission spectra of the sensing membrane incorporating NBTMB exposed to the NaOAc–HOAc buffer solution (pH 4.0) containing different concentrations of 2,4-dinitrophenol; the fluorescence spectra were recorded at λex = 328 nm and λem = 350–630 nm. Decreases in fluorescence at the emission maxima of 399 and 460 nm were observed as the concentration of 2,4-dinitrophenol increased. This illustrates that the sensing membrane can be used for the assay of nitrophenol in aqueous solution. From Fig. 2, it can be stated that the response of the sensing
membrane to 2,4-dinitrophenol at 399 nm is more sensitive than that at 460 nm. In our subsequent experiments, quantitative measurements were made at λex = 328 nm and λem = 399 nm.
 |
| | Fig. 2 Fluorescence emission spectra of the NBTMB sensing membrane exposed to different concentrations of 2,4-dinitrophenol solutions: (0), 0; (1), 7.80 × 10−7; (2), 2.62 × 10−6; (3), 5.26 × 10−6; (4), 9.0 × 10−6; (5), 1.90 × 10−5; (6), 3.0 × 10−5; (7), 4.0 × 10−5; (8), 6.0 × 10−5; (9), 1.20 × 10−4; (10), 2.00 × 10−4; (11), 1.00 × 10−3 mol L−1. | |
Principle of operation
In order to explain the aforementioned phenomena complex formation, the association between NBTMB in the membrane and nitrophenol in aqueous solution can be represented by the following equilibrium: | |  | (1) |
where A and B represent nitrophenol and NBTMB, respectively, and (aq) and (mem) represent the aqueous solution phase and membrane phase. We can write the following equation:24| |  | (2) |
where CB is the total concentration of NBTMB in the membrane, [A] is the concentration of nitrophenol in aqueous solution, K is the equilibrium constant of the reaction described by eqn. (1), which is related to the distribution coefficient and complex formation constant, and α (relative fluorescence value)
is the ratio of the free NBTMB concentration, [B], to the total amount of NBTMB present in the membrane, CB. α can be expressed as:| |  | (3) |
where F0 and F1 are the limiting fluorescence intensities when the sensing membrane is contacted with the blank buffer solution and 8.0 × 10−4 mol L−1 of 2,4-dinitrophenol solution, respectively, and F is the fluorescence intensity of the sensing membrane exposed to different concentrations of 2,4-dinitrophenol solution. Obviously, eqn. (2) provides the basis for the quantitative determination of 2,4-dinitrophenol. The experimental data were fitted to eqn. (2) by changing the ratio of m to n and adjusting the overall equilibrium constant K. Fig.
3 shows the fitted curves representing the experimental data for 2,4-dinitrophenol. The curve referring to the 1∶1 complex ratio and K = 5.0 × 104 L mol−1 is the best one fitted to the experimental data (curve 3). The best fitting curve can serve as the calibration curve for the determination of 2,4-dinitrophenol.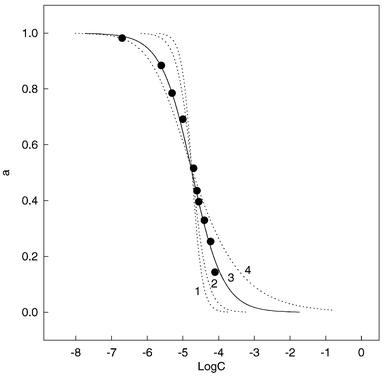 |
| | Fig. 3 Fitting experimental data (●) to eqn. (2): (1), m = 3, n = 1, K = 1.7 × 1014; (2), m = 2, n = 1, K = 3.0 × 109; (3), m = 1, n = 1, K = 5.0 × 104(best fitting); (4), m = 1, n = 2, K = 5.5 × 106. | |
Characteristics of the sensor
Optimization of membrane composition. The sensing membrane responses to nitrophenols may be changed by variation of the compositions of the membrane cocktail. This can be seen in the variation of the sensor characteristics (linear range and response slope) due to the different concentrations of NBTMB. The fluorescence quenching efficiencies (F0/F) for four membranes (M1, M2, M3 and M4, see Table 1) at λex = 328 nm and λem = 399 nm are plotted as functions of the 2,4-dinitrophenol concentrations at pH 4.0, where F0 and F are the fluorescence intensities of the sensing membranes in the absence and presence of the analyte, respectively. Obviously, sensing membrane M2 emerged as the best in terms of response slope and linear range for the determination of 2,4-dinitrophenol.Changing the type of the plasticizer is another way to alter the sensor characteristics. Appropriate plasticizers should be selected so as to obtain a transparent and flexible membrane, which has the maximum response to the analyte. Sensing membranes made of different plasticizers such as tricresyl phosphate, bis(2-ethylhexyl) sebacate, dibutyl phthalate and didecyl phthalate were prepared; the membrane containing bis(2-ethylhexyl)sebacate (DOS) performed the best response to the 2,4-dinitrophenol. The optimum ratio of DOS to PVC was 2:1 (w/w). The optimum sensing membrane shows a linear response to 2,4-dinitrophenol in the range 2.00 × 10−7–6.00 × 10−5 mol L−1.
Effect of pH. Before fully characterizing the sensing membrane, the effect of pH on the quenching efficiency was investigated. Fig. 4 shows the effect of pH on the fluorescence quenching efficiency, (F0/F) − 1. The fluorescence measurements were performed for 1.05 × 10−5 mol L−1 of 2,4-dinitrophenol at different pH values. From the results, it can be stated that the quenching efficiency changes with pH: fluorescence quenching increases as the pH increases from 2.03 to 4.92, whereas quenching decreases on going from pH 5.42 to 11.23. These results seem to be related to the extent of ionization of 2,4-dinitrophenol at different pH values. The pKa value of 2,4-dinitrophenol is 4.11;40 at pH >5.42, 2,4-dinitrophenol tends to dissociate more completely and is not easily extracted from the aqueous solution phase
into the organic membrane phase. After the pH has reached a relative low value, the ionization of 2,4-dinitrophenol will be inhibited so that the quenching remains constant. On the other hand, too high an acidity favors the extraction of H+ from aqueous solution into the membrane phase, and the fluorescence intensity value of the blank membrane decreases. In subsequent experiments, a pH 4.0 buffer solution was selected as optimum.
 |
| | Fig. 4 Influence of solution pH on the membrane response to exposure to 1.05 × 10−6 mol L−1 2,4-dinitrophenol. | |
Reproducibility, reversibility and response time. It would be desirable for a sensor to have good reproducibility, reversibility and a short response time. The reproducibility and reversibility of the sensing membrane were evaluated by repeatedly switching the membrane into three standard solutions of different concentrations. Fig. 5 shows the fluorescence intensities at λex = 328 nm and λem = 399 nm versus recording time for the sensing membrane when it was exposed to repeated concentration step changes among 5.26 × 10−6, 1.05 × 10−5 and 1.90 × 10−5 mol L−1 of 2,4-dinitrophenol in NaOAc–HOAc buffer solution. The relative standard deviations (RSDs) were found to be 1.45% (5.26 × 10−6 mol L−1 of 2,4-dinitrophenol, n = 4), 0.66% (1.05 × 10−5
mol L−1 of 2,4-dinitrophenol, n = 4) and 1.59% (2.10 × 10−5mol L−1 of 2,4-dinitrophenol, n = 4). The results show high reproducibility and reversibility of the optical signals. From Fig. 5. it can be seen that the response time (t95%) of this sensing membrane for 2,4-dinitrophenol is <1.5 min.
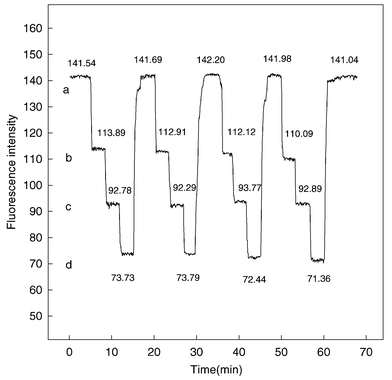 |
| | Fig. 5 Fluorescence intensities of the optode membrane versus time at λex = 328 nm and λem = 399 nm after several repeated concentration step changes among (a), 0; (b), 5.26 × 10−6; (c), 1.05 × 10−5; and (d), 1.90 × 10−5 mol L−1 2,4-dinitrophenol. | |
Short-term stability and lifetime. The stability of the sensor was tested by using 1.05 × 10−5 mol L−1 2,4-dinitrophenol solution. The fluorescence response of the sensing membrane in contact with 1.05 × 10−5 mol L−1 2,4-dinitrophenol was recorded over a period of 10 h at intervals of 30 min. The mean fluorescence intensity and standard deviation were 92.37 ± 3.23 (n = 21, RSD = 3.48%). This shows that the sensing membrane has good short-term stability. During the measurement of the sensing membrane over 3 weeks, the fluorescence intensity of the membrane at λex = 328 nm and λem = 399 nm decreased by 6%. Apparently, the lifetime of the sensing membrane is acceptable for continuous analytical applications.
Sensor response to different nitrophenols. To define the response of the sensor to other nitrophenols, the sensing membrane was subjected to different concentrations of o-nitrophenol, m-nitrophenol, p-nitrophenol, 2,4-dinitrophenol and picric acid separately with a contact time of 2 min. In Fig. 6, the membrane response, α, at pH 4.0 is plotted as a function of the logarithm of concentration of all the nitrophenols. The curve fitting for the experimental points was calculated from eqn. (2) assuming a 1∶1 complex ratio. From the α values of the different nitrophenols at the same concentration, it can be seen that the response of the sensing membrane towards nitrophenols decreased in the order 2,4-dinitrophenol > picric acid > o-nitrophenol > p-nitrophenol > m-nitrophenol. This discrimination may be attributed to the different structures of the analytes.
2,4-dinitrophenol, picric acid and o-nitrophenol can form intramolecular hydrogen bonds which may encounter less steric hindrance and stabilize the complex structure as it interacts with NBTMB. 2,4-Dinitrophenol has a direct and relatively strong ion-pair interaction with NBTMB. On the other hand, at pH 4.0, picric acid tends to dissociate more completely and is mainly present in anionic form, which in turn will weaken its migration process from the bulk of the solution to the organic membrane phase and the response decreases.![Membrane response (α) at λex = 328 nm and λem = 399 nm as a function of log[nitrophenol] at pH 4.0. The curve fittings for experimental data were calculated from eqn. (2) (n∶m = 1∶1). ●, Experimentally observed data points.](/image/article/2002/AN/b106346b/b106346b-f6.gif) |
| | Fig. 6 Membrane response (α) at λex = 328 nm and λem = 399 nm as a function of log[nitrophenol] at pH 4.0. The curve fittings for experimental data were calculated from eqn. (2) (n∶m = 1∶1). ●, Experimentally observed data points. | |
Selectivity. Substances that can partition into the membrane and react with NBTMB will influence the measurements. The effects of other potential interferents such as aniline, phenol and some ions were tested for their possible co-occurrences with nitrophenol in environmental samples. The tolerance limit was set as the concentration of interferent that produced a variation in the apparent recovery of 1.90 × 10−6 mol L−1 of 2,4-dinitrophenol of <±5% relative error. Table 2 summarizes the results obtained. It can be seen that large amounts of alkali metal ions and Mn2+, Ni2+, Co2+ and Cu2+ do not interfere. Among the rest of the ions tested, Pb2+, Hg2+ and Cr2O72− can be tolerated in up to 50–80-fold molar excess, and Fe3+ up to fivefold. Fe2+ shows
serious interference in the determination of nitrophenol, which could be avoided with the addition of orthophosphoric acid to the sample solution.36
Table 2 Effect of different interferents on the fluorescence intensity of the sensing membranea
| Interferent | Concentration/mol L−1 | Fluorescence intensity,F2 | Fluorescence change, ΔF = (F1
− F2)b | Relative error, ΔF/F1
× 100 |
|---|
| |
|---|
| Each sample solution contained a fixed concentration of 2,4-dinitrophenol of 1.90 × 10−6 mol L−1. The fluorescence intensities were recorded after the sample solutions were contacted with the sensing membrane for 2 min. F1 and F2 are the fluorescence intensities of the sensing membrane contacted with 1.90 × 10−6 mol L−1 2,4-dinitrophenol solution without and with the interferents, respectively. |
|---|
| NH4+ | 7.91 × 10−2 | 131.8 | −2.45 | −1.89 |
| Na+ | 7.91 × 10−2 | 125.1 | 4.25 | 3.29 |
| Ba2+ | 8.00 × 10−2 | 128.2 | 1.15 | 0.89 |
| Ca2+ | 8.03 × 10−2 | 126.4 | 2.95 | 2.28 |
| Mn2+ | 8.08 × 10−3 | 130.3 | −0.95 | −0.73 |
| Ni2+ | 8.05 × 10−2 | 133.2 | −3.85 | −2.98 |
| Hg2+ | 1.60 × 10−4 | 125.9 | 3.45 | 2.67 |
| Co2+ | 2.00 × 10−3 | 124.9 | 4.45 | 3.44 |
| Fe2+ | 1.00 × 10−6 | 123.0 | 6.35 | 4.91 |
| Fe3+ | 1.00 × 10−5 | 122.9 | 6.45 | 4.99 |
| Pb2+ | 1.02 × 10−4 | 124.7 | 4.56 | 3.59 |
| Cu2+ | 8.00 × 10−3 | 127.6 | 1.75 | 1.35 |
| F− | 8.80 × 10−3 | 133.4 | −4.05 | −3.13 |
| Cl− | 8.00 × 10−3 | 131.8 | −2.45 | −1.89 |
| Br− | 7.07 × 10−3 | 126.3 | 3.50 | 2.36 |
| I− | 3.90 × 10−4 | 125.5 | 3.85 | 2.98 |
| SO42− | 6.02 × 10−2 | 125.8 | 3.55 | 2.74 |
| ClO4− | 2.01 × 10−4 | 126.5 | 2.85 | 2.20 |
| NO2− | 1.45 × 10−4 | 125.3 | 4.05 | 3.13 |
| SCN− | 8.46 × 10−4 | 123.9 | 5.45 | 4.21 |
| C2O42− | 4.03 × 10−4 | 126.8 | 2.55 | 1.97 |
| PO43− | 1.00 × 10−2 | 129.5 | −0.15 | −0.12 |
| Cr2O72− | 1.15 × 10−4 | 123.1 | 6.25 | 4.83 |
| Ethanol | 1.83 × 10−1 | 132.4 | −3.05 | −2.36 |
| Methanol | 1.95 × 10−1 | 130.9 | −1.55 | −1.20 |
| Acetone | 1.21 × 10−2 | 134.6 | −5.25 | −4.06 |
| Aniline | 7.80 × 10−3 | 130.4 | −1.05 | −0.81 |
| Phenol | 1.00 × 10−2 | 124.5 | 4.85 | 3.75 |
The common anions and organic substances had negligible effects even at levels >103 times that of 2,4-dinitrophenol.
Application
The proposed method was tested by applying it to the direct determination of 2,4-dinitrophenol in prepared water samples in the presence of other interferents and to the indirect determination of some drugs such as cinchonine. The prepared water samples with different amounts of 2,4-dinitrophenols added were analyzed and the recovery was 95.2–105% (Table 3).
Table 3 Analytical results for 2,4-dinitrophenol in three prepared water samples
| Sample | Composition of sample/10−6 mol L−1 | Determineda/10−6 mol L−1 | Recovery (%) |
|---|
| |
|---|
| Mean values ±
s of three determinations. |
|---|
| 1 | NaCl, 10; KBr,20; NH4Cl, 10; BaCl2, 20; NiCl2, 20; ZnCl2, 20; CuSO4, 10; KClO4, 10; KSCN, 10; K2HPO4, 20; ethanol, 20; phenol, 10; acetone, 20; aniline, 20; 2,4-dinitrophenol, 0.80. | 0.84 ± 0.045 | 105.00 |
| |
| |
| |
| |
| |
| 2 | NaCl, 20; KBr, 10; NH4Cl, 20; BaCl2, 20; NiCl2, 10; ZnCl2, 10; CuSO4, 5; KClO4, 20; KSCN, 10; K2HPO4, 20; ethanol, 40; phenol, 10; acetone, 20; aniline, 10; 2,4-dinitrophenol, 40.0. | 38.08 ± 1.28 | 95.20 |
| |
| |
| |
| |
| |
| 3 | NaCl, 20; KBr, 30; NH4Cl, 20; BaCl2, 10; NiCl2,20; ZnCl2, 20; CuSO4, 20; KClO4, 10; KSCN, 20; K2HPO4, 10; ethanol, 20; phenol, 20; acetone, 10; aniline, 30; 2,4-dinitrophenol, 80.0. | 78.16 ± 4.52 | 97.70 |
| |
| |
| |
| |
| |
The indirect method for the determination of cinchonine is based on its quantitative precipitation with picric acid. The determination of cinchonine was carried out by the following procedure. About 0.1 g of sample was dissolved in 2.0 × 10−3 mol L−1 H2SO4 to give 25 mL of solution. To this solution, 5 mL of 2.0 × 10−4 mol L−1 picric acid were added with stirring. The solution was filtered and the filtrate was diluted to 50 mL with NaOAc–HOAc buffer solution. The picric acid content of the filtrate was determined by using the described method. For 1.54 × 10−4 mol L−1 cinchonine, the measured mean concentration was 1.71 × 10−4 mol L−1. Six repeated measurements for different aliquots from the sample solution gave a standard deviation of 1.02 × 10−5 mol L−1. The
results were in agreement with those obtained by another method.26
Acknowledgements
This work was supported by the National Outstanding Youth Foundation of China (29875110), Joint Research Fund for Overseas Chinese and Hong Kong Youth Scholar of the National Natural Science Foundation of China (20028506), Natural Science Foundation of Hunan Province (00JKY1011) and Key Project Foundation of the China Education Ministry (2000-156).References
- P. R. W. Baker, Analyst, 1954, 79, 289 RSC.
- M. Tarasiewicz and H. Basinska, Talanta, 1974, 21, 425 CrossRef.
- Shanghai Sanitation and Antiepidemic, Environmental Protection Examination, Shanghai Science and Technology Press, Shanghai, 1977 Search PubMed.
- S. S. Chan and T. X. Wang, Environ. Sci., 1985, 6, 71 Search PubMed.
- M. Kadota, M. Imanaka, K. Ikegawa, K. Kumashiro, T. Mori, S. Suzuki and H. Nakawaza, J. Food Hyg. Soc. Jpn., 1996, 37, 48 Search PubMed.
- K. Wrobel, E. M. C. Urbina and J. M. Romero, J. Pharm. Biomed. Anal., 2000, 22, 295 CrossRef CAS.
- T. J. Diaz, A. Guiberteau, J. M. Ortiz, M. D. Lepez and F. Salinas, Chromatographia, 2001, 53, 40 Search PubMed.
- T. P. Hadjiioannou and E. P. Diamandis, Anal. Chim. Acta, 1977, 94, 443 CrossRef.
- E. P. Diamandis and T. P. Hadjiioannou, Anal. Chim. Acta, 1981, 123, 341 CrossRef CAS.
- V. N. Maistrenko, S. V. Sapepnikova, F. K. Kudasheva and F. V. Amirkhanova, J. Anal. Chem., 2000, 55, 586 Search PubMed.
- K. Maeda, S. Nagami, Y. Yoshida, H. Ohde and S. Kihara, J. Electroanal. Chem., 2001, 496, 124 CrossRef CAS.
- A. W. Czarnik, Acc. Chem. Res., 1994, 27, 302 CrossRef CAS.
- B. Valeur and E. Bardez, Chem. Br., 1995, 216 Search PubMed.
- S. West, S. Ozawa, K. Seiler, S. S. S. Tan and W. Simon, Anal. Chem., 1992, 64, 533 CrossRef CAS.
- R. H. Yang, K. M. Wang, D. Xiao and X. H. Yang, Fresenius’ J. Anal. Chem., 2000, 367, 429 CrossRef CAS.
- L. L. Vasileva, A. S. Kushkova, S. M. Repinskii and V. N. Fedorinin, J. Anal. Chem., 2000, 55, 636 Search PubMed.
- J. S. Do and W. B. Chang, Sens. Actuators B, 2001, 72, 101 CrossRef.
- F. P. Milanovich, D. G. Garvis, S. M. Angel and S. M. Klainer, Anal. Instrum., 1986, 15, 136 Search PubMed.
- K. M. Wang, H. H. Zeng and R. Q. Yu, Chem. J. Chin. Univ., 1994, 15, 836 Search PubMed.
- W. H. Chan, A. W. M. Lee, J. Z. Lu and X. J. Wu, Anal. Chim. Acta, 1998, 370, 259 CrossRef CAS.
- G. Walkup and B. Imperiali, J. Am. Chem. Soc., 1997, 118, 3443 CrossRef CAS.
- S. Turiainen, M. Karp, W. Chang and M. Virta, Biosens. Bioelectron., 1998, 13, 931 CrossRef.
- C. Jian and W.
R. Seitz, Anal. Chim. Acta, 1990, 273, 265 CrossRef CAS.
- H. H. Zeng, K. M. Wang, C. L. Liu and R. Q. Yu, Talanta, 1993, 40, 1569 CrossRef CAS.
- Y. Wang, K. M. Wang, G. L. Shen and R. Q. Yu, Talanta, 1997, 44, 1319 CrossRef CAS.
- X. Yang, C. G. Niu, G. L. Shen and R. Q. Yu, Analyst, 2001, 126, 349 RSC.
- R. H. Yang, K. M. Wang, D. Xiao and X. H. Yang, Analyst, 2000, 125, 877 RSC.
- G. D. Santis, L. Fabbrizzi, M. Licchelli, A. Poggi and A. Taglietti, Angew. Chem., Int. Ed. Engl., 1996, 35, 202 CrossRef.
- K. M. Wang, K. Seiler, B. Rusterholz and W. Simon, Analyst, 1992, 117, 57 RSC.
- R. H. Yang, K. M. Wang, D. Xiao and X. H. Yang, Anal. Chim. Acta, 2000, 404, 205 CrossRef CAS.
- K. M. Wang, K. Seiler, W. E. Morf, U. E. Spichiger, W. Simon, E. Lindner and E. Pungor, Anal. Sci., 1990, 6, 715 Search PubMed.
- P. D. Thomas and M. J. Poznanzki, Anal. Biochem., 1990, 188, 228 CrossRef CAS.
- K. J. Williams, R. Rosenstein and R. P. Smith, Clin. Chim. Acta, 1985, 145, 113 CrossRef CAS.
- T. Shiobara, N. Teshima, M. Kurihara, S. Nakano and T. Kawashima, Talanta, 1997, 49, 1083 CrossRef CAS.
- R. H. Yang, K. M. Wang, D. Xiao and X. H. Yang, Fresenius’ J. Anal. Chem., 2000, 368, 797 CrossRef CAS.
- F. B. Serrat, Talanta, 1994, 41, 2091 CrossRef CAS.
- K. M. Attar, P. West and W. Arabian, J. Sci. Eng., 1985, 10, 107 Search PubMed.
- H. He, H. Li, G. Mohr, B. Kovács, T. Werner and O. S. Wolfbeis, Anal. Chem., 1993, 65, 123 CrossRef CAS.
- W. H. Chan, A. W. M. Lee, Y. S. Lam and K. M. Wang, Anal. Chim. Acta, 1997, 351, 197 CrossRef CAS.
- G. H. Aylward and T. J. V. Findlay, SI Chemical Data, Wiley, New York, 2nd edn., 1974, reprinted 1982, p. 81 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2002 |
Click here to see how this site uses Cookies. View our privacy policy here. 







![Membrane response (α) at λex = 328 nm and λem = 399 nm as a function of log[nitrophenol] at pH 4.0. The curve fittings for experimental data were calculated from eqn. (2) (n∶m = 1∶1). ●, Experimentally observed data points.](/image/article/2002/AN/b106346b/b106346b-f6.gif)

