Cooperativity of binuclear Zn(II) complexes of bisimidazolyl ligands in the hydrolysis of bis(2,4-dinitrophenyl) phosphate in aqueous solution
Shin-ichi
Kondo
a,
Koichi
Shinbo
a,
Tatsuya
Yamaguchi
a,
Kitaro
Yoshida
b and
Yumihiko
Yano
*a
aDepartment of Chemistry, Gunma University, Kiryu, Gunma, 376-8515, Japan. E-mail: yano@chem.gunma-u.ac.jp
bDepartment of Chemistry, Saitama Medical School, Moroyama, Saitama, 350-0496, Japan
First published on 18th December 2000
Abstract
Hydrolysis of bis(2,4-dinitrophenyl) phosphate (BDNPP) by Zn(II) with ligands bearing plural bis(imidazol-2-yl) groups was kinetically studied in aqueous solution. The binuclear Zn(II) complex of 1,3-bis(diimidazol-2-ylhydroxymethyl)benzene (2a) was found to be most effective for hydrolysis of BDNPP (1.2 × 103-fold). ESI-MS study revealed formation of 2a·Zn(II)2 in MeOH–H2O, suggesting that the rate acceleration is due to so called double Lewis acid activation. The pH–rate profile showed that the pKa of the active species is around 7.5. The rate accelerations by the complexes of 2a with other metal ions were 4.3-fold for Ni(II), 7.1-fold for Co(II), and 1.9 × 102-fold for La(III).
Introduction
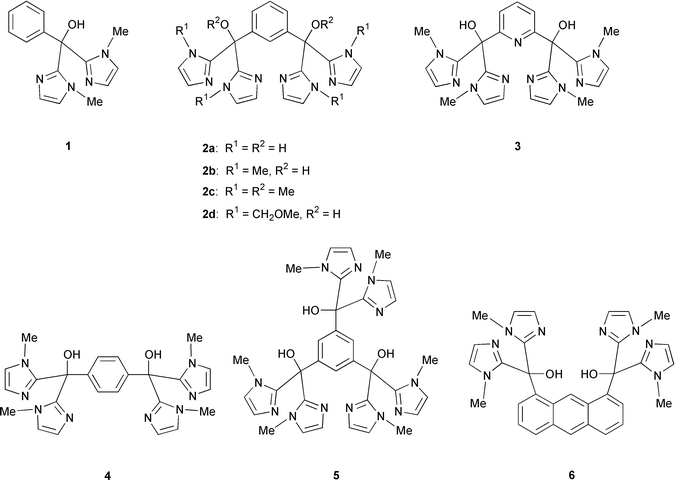
Phosphodiester bonds are known to be cleaved by nucleases possessing two (or more) divalent metal ions located in 3–5 Å in the active site.1 Binuclear metal complexes have attracted considerable attention from the viewpoint of two-metal ion catalysis,1a,2 and the activities for hydrolytic cleavage of phosphodiester bonds have been extensively investigated.3 The metal ions are considered to activate the P–O bond and water bound to the metal ion, and to stabilize the transition state and the leaving group by acting as a Lewis acid. However, the hydrolytic activities are dependent on the kind of metal ions and the ligand structure. Recently, Reinhoudt et al. have reported that calix[4]arene functionalized with two bisimidazolyl groups shows a large rate enhancement for transesterification of 2-hydroxypropyl-p-nitrophenyl phosphate with Cu(II), but not with Zn(II).3a,c On the other hand, calix[4]arene functionalized with 2,6-bis(aminomethyl)pyridyl groups exhibits a much larger activity with Zn(II) than with Cu(II).3b These results indicate that combination of a ligand and a metal ion is important for the design of binuclear metal complexes for hydrolysis of phosphodiesters. More importantly, Reinhoudt et al. have mentioned that flexibility of the ligands is responsible for the catalytic activity.Although there are imidazolyl ligands of histidines in the active sites of many native nucleases,1 binuclear Zn(II) complexes of imidazolyl ligands have been scarcely studied in model systems.4 For example, Trögler et al. have reported that Zn(II) complexes of tetrakis(1-methylimidazol-2-yl) derivatives show no cooperative rate enhancements for hydrolysis of bis(4-nitrophenyl) phosphate, probably due to the ligand structure being either too rigid or too flexible.4b This prompted us to design ligands bearing bisimidazolyl moieties as shown in Chart 1. In particular, we focused our attention on the substituent effect on N(1) of the imidazolyl groups of the ligands, since 1-methylimidazole is usually used. We report herein that a binuclear Zn(II) complex of 1,3-bis(diimidazol-2-ylhydroxymethyl)benzene (2a) shows a remarkable rate acceleration (1.2 × 103-fold) for hydrolysis of BDNPP in aqueous buffer solution.
 | ||
| Chart 1 | ||
Results and discussion
Synthesis of the ligands
The ligands were synthesized from 2-lithio-1-methylimidazole or 2-lithio-1-(methoxymethyl)imidazole with the corresponding carboxylic acid esters in THF according to literature procedures.4b,5 Diethyl anthracene-1,8-dicarboxylate was prepared from 1,8-dichloroanthraquinone according to the literature.6Metal binding
The binding ability of the ligands 2a, 2b, and 2d for Zn(II) was spectrophotometrically examined in aqueous solution. Plots of the absorption changes of 2 at 249.3 nm upon addition of Zn(II) are shown in Fig. 1. The absorption changes of 2a and 2b are saturated at [Zn(II)]/[2] = 2, indicating formation of binuclear Zn(II) complexes of 2a and 2b, whereas the absorption changes of 2d are saturated at [Zn(II)]/[2d] = 1. Potentiometric pH titrations were performed as follows. An aqueous solution of 2 (2.0 × 10−4 mol dm−3) in 0.1 mol dm−3 KNO3 was acidified with HNO3 (8.0 × 10−4 mol dm−3) and titrated with KOH (2.0 × 10−2 mol dm−3) under a stream of humidified N2 at 25 °C in the absence (for determination of ligand protonation constants) and presence of Zn(NO3)2 (for determination of binding constant and deprotonation constants of Zn(II) bound water). pH values were recorded with a Fisher Scientific Accumet AC-15 pH meter. However, the protonation and binding constants were not obtained accurately because of too slow an equilibrium. Spectrophotometric titration of 3 and Zn(II) showed the 1∶1 complex formation of 3, which could be explained by involvement of the pyridine N of 3 as a fifth ligand. Meanwhile, the binding constants of 1 and diimidazol-2-ylmethane with Zn(II) are known to be 9 × 104 and 5.4 × 105 dm3 mol−1, respectively.4a,5a![Titration of 2 with Zn(II). [2] = 4.0 × 10−4 mol dm−3, [Zn(NO3)2·6H2O] = 0–2.4 × 10−3 mol dm−3, pH 7.0 (0.05 mol dm−3 HEPES, I = 0.1 with KNO3), at 25 °C.](/image/article/2001/P2/b002395g/b002395g-f1.gif) | ||
| Fig. 1 Titration of 2 with Zn(II). [2] = 4.0 × 10−4 mol dm−3, [Zn(NO3)2·6H2O] = 0–2.4 × 10−3 mol dm−3, pH 7.0 (0.05 mol dm−3 HEPES, I = 0.1 with KNO3), at 25 °C. | ||
The complex formation of 2 and Zn(II) was also examined in MeCN and 50% MeOH–H2O by electron spray ionization mass spectroscopy (ESI-MS). In MeCN, molecular peaks corresponding to binuclear Zn(II) complexes were observed: m/z 761 ([2a·Zn(II)2·(NO3−)3·MeCN]+), m/z 776 ([2b·Zn(II)2· (NO3−)3]+), and m/z 896 ([2d·Zn(II)2·(NO3−)3]+), respectively. The isotope patterns were in good agreement with the theoretical ones. In 50% MeOH–H2O, however, the peak for the binuclear Zn(II) complex was observed only for 2a, whereas the peaks corresponding to mononuclear complexes, [2b·Zn(II)· NO3−]+ and [2d·Zn(II)·NO3−]+ were observed. These results suggest that the binding ability of 2a to Zn(II) is stronger than that of 2b or 2d in aqueous solution in order of bulkiness of substituents.
Hydrolytic activity
Hydrolytic activities of the ligands in the presence of the metal ions were kinetically evaluated for hydrolysis of BDNPP in aqueous solution. Pseudo-first-order rate constants (kobs) were determined spectrophotometrically by following absorption increases of 2,4-dinitrophenolate at 400 nm at 25 °C in aqueous solution.The effects of the ligands on kobs were firstly examined in the presence of Zn(II) at pH 7.0. The rate constants and relative rates are listed in Table 1. It should be noted that the rate constants were calculated within 10% reaction, although the following hydrolysis of 2,4-dinitrophenyl phosphate was confirmed to be about 10 times slower than that of BDNPP under the conditions of Table 1. As expected, the rate accelerations were dependent on the structure of the ligands. In a series of 2, the rates are sensitive to the N(1)-substituent of the imidazole groups (2a, 2b, and 2d), but not to the O-substituents (2b and 2c), suggesting that hydroxy groups of the ligands are not involved during the hydrolysis. The activity of the ligand 5 bearing three bis(1-methylimidazol-2-yl)hydroxy units is almost the same as that of 2b, indicating no participation of the third bisimidazolyl unit. No effect of 3 can be explained by a 1∶1 complexation, and no effect of 6 may be explained by unsuitable positioning of two Zn(II)’s. Since the m-phenylene spacer series (2) was effective, further kinetic study was undertaken by employing 2.
| Ligands | k obs/s−1 | Relative rates |
|---|---|---|
| [BDNPP] = 5.0 × 10−5 mol dm−3, [ligand] = 2.5 × 10−4 mol dm−3, [Zn(NO3)2·6H2O] = 1.0 × 10−3 mol dm−3, pH 7.0 (0.05 mol dm−3 HEPES, I = 0.1 with KNO3), 25 °C. | ||
| None | 1.7 × 10−7 | 1.0 |
| Zn(II) | 1.0 × 10−6 | 6.0 |
| 1 + Zn(II) | 1.8 × 10−6 | 11 |
| 2a + Zn(II) | 2.0 × 10−4 | 1200 |
| 2b + Zn(II) | 3.5 × 10−5 | 210 |
| 2c + Zn(II) | 3.3 × 10−5 | 200 |
| 2d + Zn(II) | 6.8 × 10−6 | 40 |
| 3 + Zn(II) | 6.3 × 10−7 | 3.7 |
| 4 + Zn(II) | 1.1 × 10−5 | 65 |
| 5 + Zn(II) | 4.8 × 10−5 | 280 |
| 6 + Zn(II) | 6.8 × 10−7 | 4.0 |
The effects of the concentration of Zn(II) are shown in Fig. 2. The rate for 2a increases abruptly at [Zn(II)] = 5.0 × 10−4 mol dm−3 (2 molar excess over [2a]) and reaches saturation, suggesting that the reactive species is binuclear complex 2a·[Zn(II)]2. A bulkier substituent on the N(1) of the imidazolyl groups decreases the rates (2a > 2b = 2c > 2d). This order could be explained by steric hindrance of the N-substituents for the metal complexation and/or the subsequent coordination to the substrate BDNPP.
![Plots of kobsvs. [Zn(II)]. [BDNPP] = 5.0 × 10−5 mol dm−3, [ligand] = 2.5 × 10−4 mol dm−3, [Zn(NO3)2·6H2O] = 0–2.0 × 10−3 mol dm−3, pH 7.0 (0.05 mol dm−3 HEPES, I = 0.1 with KNO3), at 25 °C.](/image/article/2001/P2/b002395g/b002395g-f2.gif) | ||
| Fig. 2 Plots of kobsvs. [Zn(II)]. [BDNPP] = 5.0 × 10−5 mol dm−3, [ligand] = 2.5 × 10−4 mol dm−3, [Zn(NO3)2·6H2O] = 0–2.0 × 10−3 mol dm−3, pH 7.0 (0.05 mol dm−3 HEPES, I = 0.1 with KNO3), at 25 °C. | ||
The effects of pH on the rates were examined by employing 2b·[Zn(II)]2 in buffer solutions containing MeCN (33.3% v/v) to prevent precipitation. As shown in Fig. 3,† the rate is first-order with respect to [OH−] and reaches saturation at pH ca. 7.5. This implies that the hydrolysis proceeds via an attack of Zn(II) bound hydroxide ion, because the pKa of 1·Zn(II) bound water is reported to be 7.4.4a
![Plots of kobsvs. pH. [BDNPP] = 5.0 × 10−5 mol dm−3, [2b] = 2.5 × 10−4 mol dm−3, [Zn(NO3)2·6H2O] = 2.5 × 10−3 mol dm−3, 0.05 mol dm−3 HEPES, I = 0.1 with KNO3 with MeCN (33.3%, v/v), at 25 °C.](/image/article/2001/P2/b002395g/b002395g-f3.gif) | ||
| Fig. 3 Plots of kobsvs. pH. [BDNPP] = 5.0 × 10−5 mol dm−3, [2b] = 2.5 × 10−4 mol dm−3, [Zn(NO3)2·6H2O] = 2.5 × 10−3 mol dm−3, 0.05 mol dm−3 HEPES, I = 0.1 with KNO3 with MeCN (33.3%, v/v), at 25 °C. | ||
The activities of other metal ions such as Ni(II), Co(II) and La(III) with 2a for hydrolysis of BDNPP were briefly examined (Table 2), indicating Zn(II) to be the most effective. It should be emphasized that the combination of Zn(II) and the bisimidazolyl ligand shows a cooperative rate acceleration for hydrolysis of phosphate diesters.
| Metal ion | k obs/s−1 | Relative rates |
|---|---|---|
| [BDNPP] = 5.0 × 10−5 mol dm−3, [2a] = 2.5 × 10−4 mol dm−3, [metal ion] = 1.0 × 10−3 mol dm−3, pH 7.0 (0.05 mol dm−3 HEPES, I = 0.1 with KNO3), 25 °C. | ||
| Zn(II) | 2.0 × 10−4 | 1200 |
| Ni(II) | 7.3 × 10−7 | 4.3 |
| Co(II) | 1.2 × 10−6 | 7.1 |
| La(III) | 3.3 × 10−5 | 190 |
The data obtained indicate cooperativity of two metal ions in the presence of ligands. Namely, one Zn(II) bound to the ligand is associated with a negative charge of BDNPP to form a complex, and the second Zn(II) provides a ligated hydroxide ion which acts as a nucleophile within the complex, wherein either of the Zn(II)’s assists the nucleophilic attack by acting as a Lewis acid. Of particular interest in this work is that the largest rate acceleration is observed for the binuclear Zn(II) complex of 1-unsubstituted imidazolyl ligand 2a. A CPK model study of 2a·[Zn(II)]2 suggests that there is some flexibility compared with 2b·Zn(II)2 and 2d·Zn(II)2, the 2a complex being able to adopt a position suitable for the two metal ion mechanism.
Conclusion
In summary, we have demonstrated that a binuclear Zn(II) complex of 2a shows a considerable rate acceleration for hydrolysis of activated phosphate diester in aqueous solution. To our knowledge, it is the first example of a combination of Zn(II) and imidazolyl ligands which shows cooperative rate accelerations in the hydrolysis of phosphate diesters. We believe that the present results would be useful for the design of ligands using imidazole groups.Experimental
THF was purified by distillation from Na–benzophenone. MeCN was dried and distilled over calcium hydride. Other reagents were of analytical or special grade. M(NO3)2·6H2O (M: Zn, Co, Ni) and La(NO3)3·6H2O were used. Buffer solutions were prepared in deionized and distilled water by employing 0.05 mol dm−3 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) with I = 0.1 (KNO3). UV–vis spectra were recorded on Shimadzu UV-2200A and JASCO Ubest-560 spectrometers with a thermal regulator (±0.5 °C). 1H NMR spectra were measured on a Varian Gemini-200 spectrometer. Electron spray ionization mass spectra (ESI-MS) were recorded on a Perkin-Elmer Sciex API-100 spectrometer. Column chromatography was performed by using Wakogel C-200 (silica gel, 70–250 μm, Wako Chemical Co. Ltd). Melting points are uncorrected. Elemental analyses were performed at the Center of Instrumental Analysis of Gunma University.Synthesis
Pyridinium bis(2,4-dinitrophenyl) phosphate (BDNPP) was prepared according to the literature, mp 157–158 °C (lit.,7 159–160 °C).The ligands were synthesized from the corresponding carboxylic acid esters and 2-lithio-1-methylimidazole or 2-lithio-1-(methoxymethyl)imidazole in THF under N2.5 Ligands 1 and 4 were known from the literature.5b Ligands 2b and 3 were supplied from our previous work.5c
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6 (2.03 g, 6.3 mmol) in THF (20 ml) was added dropwise. The reaction mixture was stirred for 30 min at −78 °C and 8 h at rt. After addition of brine (20 ml), the mixture was extracted with EtOAc (50 ml × 3). The organic layer was dried over Na2SO4 and evaporated to dryness in vacuo. The residue was recrystallized from EtOH. Yield 2.46 g (70%), mp 208–209 °C (Found: C, 68.10; H, 5.54; N, 19.42. C32H30N8O2·1
/4H2O requires C, 68.25; H, 5.46; N, 19.90%); δH (200 MHz, CDCl3) 3.35 (s, 12H), 6.1 (br s, 2H), 6.55 (d, 2H, J 7.0), 6.81 (s, 4H), 7.07 (s, 4H), 7.2 (m, 2H), 7.94 (2 H, d, J 8.4), 8.42 (1 H, s) and 9.27 (1 H, s).
6 (2.03 g, 6.3 mmol) in THF (20 ml) was added dropwise. The reaction mixture was stirred for 30 min at −78 °C and 8 h at rt. After addition of brine (20 ml), the mixture was extracted with EtOAc (50 ml × 3). The organic layer was dried over Na2SO4 and evaporated to dryness in vacuo. The residue was recrystallized from EtOH. Yield 2.46 g (70%), mp 208–209 °C (Found: C, 68.10; H, 5.54; N, 19.42. C32H30N8O2·1
/4H2O requires C, 68.25; H, 5.46; N, 19.90%); δH (200 MHz, CDCl3) 3.35 (s, 12H), 6.1 (br s, 2H), 6.55 (d, 2H, J 7.0), 6.81 (s, 4H), 7.07 (s, 4H), 7.2 (m, 2H), 7.94 (2 H, d, J 8.4), 8.42 (1 H, s) and 9.27 (1 H, s).
Kinetic measurements
In a cuvette containing 3 ml of buffer solution, suitable amounts of the ligand (5.0 × 10−2 mol dm−3 in MeOH or DMF![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) and a metal ion (2.5 × 10−1 mol dm−3 in H2O) were placed. After temperature equilibration for 30 min, the reaction was initiated by injection of 15 μl of BDNPP (1.0 × 10−2 mol dm−3 in MeCN). The absorption increases of 2,4-dinitrophenolate at 400 nm were followed. The rate constants were calculated within 10% reaction.
) and a metal ion (2.5 × 10−1 mol dm−3 in H2O) were placed. After temperature equilibration for 30 min, the reaction was initiated by injection of 15 μl of BDNPP (1.0 × 10−2 mol dm−3 in MeCN). The absorption increases of 2,4-dinitrophenolate at 400 nm were followed. The rate constants were calculated within 10% reaction.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.References
- (a) N. Strater, W. N. Lipcomb, T. Klabunde and B. C. Krebs, Angew. Chem., Int. Ed. Engl., 1996, 35, 2024 CrossRef; (b) D. E. Wilcox, Chem. Rev., 1996, 96, 2435 CrossRef CAS.
- T. A. Steitz and J. A. Steitz, Proc. Natl. Acad. Sci. USA, 1993, 90, 6498 CAS.
- (a) P. Molenveld, W. M. G. Stikvoort, H. Kooijman, A. L. Spek, J. F. Engbersen and D. N. Reinhoudt, J. Org. Chem., 1999, 64, 3896 CrossRef CAS; (b) P. Molenveld, J. F. Engbersen and D. N. Reinhoudt, J. Org. Chem., 1999, 64, 6337 CrossRef CAS; (c) P. Molenveld, J. F. Engbersen, H. Kooijman, A. L. Spek and D. N. Reinhoudt, J. Am. Chem. Soc., 1998, 120, 6726 CrossRef CAS; (d) S. Liu and A. D. Hamilton, Bioorg. Med. Chem. Lett., 1997, 7, 1779 CrossRef CAS; (e) M. Yashiro, A. Ishikubo and M. Komiyama, Chem. Commun., 1997, 83 RSC; (f) N. H. Williams and J. Chin, Chem. Commun., 1997, 131 RSC; (g) K. G. Ragunathan and H.-J. Schneider, Angew. Chem., Int. Ed. Engl., 1996, 35, 1219 CrossRef CAS; (h) T. Koike, M. Inoue, E. Kimura and M. Shiro, J. Am. Chem. Soc., 1996, 118, 3091 CrossRef CAS; (i) W. H. Chapman, Jr. and R. Breslow, J. Am. Chem. Soc., 1995, 117, 5462 CrossRef; (j) M. J. Young and J. Chin, J. Am. Chem. Soc., 1995, 117, 10577 CrossRef CAS; (k) M. Yashiro, A. Ishikubo and M. Komiyama, J. Chem. Soc., Chem. Commun., 1995, 1793 RSC; (l) D. H. Vance and A. W. Czarnik, J. Am. Chem. Soc., 1993, 115, 12165 CrossRef.
- (a) F. Chu, J. Smith, V. M. Lynch and E. V. Anslyn, Inorg. Chem., 1995, 34, 5689 CrossRef CAS; (b) E. A. Kesick, M. A. DeRosch, L. H. Freeman, C. L. Walton, D. F. Harvey and W. C. Trögler, Inorg. Chem., 1993, 32, 5851 CrossRef CAS; (c) R. G. Clewley, H. Slebocka-Tilk and R. S. Brown, Inorg. Chim. Acta, 1989, 157, 233 CrossRef CAS.
- (a) C. C. Tang, D. Davalian, P. Huang and R. Breslow, J. Am. Chem. Soc., 1978, 100, 3918 CrossRef CAS; (b) W. R. Tolman, S. S. Liu, J. G. Liu, J. G. Bentsen and S. J. Lippard, J. Am. Chem. Soc., 1991, 113, 152 CrossRef CAS; (c) S. Takano, Y. Yano and W. Tagaki, Chem. Lett., 1981, 1177 CAS.
- R. Golden and L. M. Stock, J. Am. Chem. Soc., 1972, 94, 3080 CrossRef CAS.
- C. A. Bunton and S. J. Farber, J. Org. Chem., 1969, 34, 767 CrossRef CAS.
Footnote |
| † Ten equivalents of Zn(NO3)2·6H2O were used for complete formation of 2b·[Zn(II)]2. The pH–rate profile for 2a·[Zn(II)]2 was complicated, probably because proton dissociation of the imidazolyl groups is involved. |
| This journal is © The Royal Society of Chemistry 2001 |
