Reaction of Fischer alkynylcarbene complexes with 1-azadiene derivatives: unexpected formation of 3,4-dihydropyridines
José Barluenga*a, Miguel Tomása, Alfredo Ballesterosa, Javier Santamaríaa, Raúl Corzo-Suárezb and Santiago García-Grandab
aInstituto Uni![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) ersitario de Química Organometálica “Enrique Moles” (Unidad Asociada al CSIC), Uni
ersitario de Química Organometálica “Enrique Moles” (Unidad Asociada al CSIC), Uni![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) ersidad de O
ersidad de O![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) iedo, 33071, O
iedo, 33071, O![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) iedo, Spain. E-mail: barluenga@sauron.quimica.uniovi.es; Fax: (34) 98 510 34 50
iedo, Spain. E-mail: barluenga@sauron.quimica.uniovi.es; Fax: (34) 98 510 34 50
bDepartamento de Química Física y Analítica, Uni![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) ersidad de O
ersidad de O![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) iedo, 33071, O
iedo, 33071, O![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) iedo, Spain
iedo, Spain
First published on 15th November 2000
Abstract
4-Amino-1-azabutadienes 2 underwent [3 + 3] cyclization with Fischer alkynylcarbene complexes of chromium and tungsten 1 to furnish high yields of substituted 3,4-dihydropyridines 3. The expected pyridine ring formation, which would result from cyclization/aromatization, does not take place. The process is thought to involve a 1,2-imidoyl group shift triggered by a 1,2-metal pentacarbonyl shift as the more characteristic steps. An X-ray diffraction experiment supports the proposed structure for the dihydropyridines.
Since their discovery in 1964 by Fischer,1 stabilized Fischer carbene complexes of Group 6 have been recognized to play an important role in the construction of a variety of 3- to 7-membered rings and acyclic compounds. The reaction can occur either on the carbene ligand wherein the metal acts as reactivity and selectivity auxiliary, or at the metal center allowing a great number of cycloadditions in the coordination sphere.2 Owing to their great potential, these complexes have frequently become also useful reagents in heterocyclic synthesis.
Particularly alkynylcarbene complexes3 are appropriate precursors of heterocycles through a sequence involving addition and cyclization. In this field, several [3 + 2],4 [4 + 2]5 and [4 + 3]6N-heterocyclizations using alkynyl Fischer carbene complexes have been accomplished. On the contrary, [3 + 3] N-heterocyclizations are much less common and only a few examples are known.7–9
On the other hand, we recently discovered a novel reaction pathway for alkenyl- and alkynyl-carbene complexes towards unsaturated substrates.6 This mechanism is exemplified in Scheme 1 for the [4 + 3] cycloaddition of alkenylcarbene complexes with 4-amino-1-azabutadienes giving azepines6a and consists in (i) 1,2 addition of the imine nitrogen to the carbene carbon (step 1) and 1,2-(OC)5M migration-promoted cyclization (step 2).
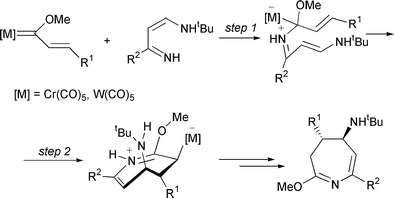 | ||
| Scheme 1 Mechanism for the synthesis of azepines from 4-amino-1-azadienes and alkenyl Fischer carbene complexes. | ||
Continuing our interest in the chemistry of azabutadiene
derivatives and Fischer carbene complexes, we report herein
the reaction of 4-amino-1-azadienes 2 with pentacarbonyl(1-methoxy-3-phenyl-2-propynylidene)-chromium
and -tungsten complexes
1 leading to dihydropyridines 3, wherein the imidoyl fragment
of the azadiene is transferred to the metal-ligand fragment.
Thus, azadiene derivatives 2 were mixed with chromium carbene
complex 1a (molar ratio 1:1) in THF at −20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and the
mixture allowed to reach 0 (for R1 = But) or 25
°C and the
mixture allowed to reach 0 (for R1 = But) or 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (for R1 = c-C6H11).
Column chromatography purification allowed us
to isolate a single adduct. Surprisingly, the expected pyridine
ring 410 was not formed at all, but substituted 3,4-dihydropyridines
3 were obtained in high yields (Scheme 2, Table 1).
Replacement of chromium complex 1a with tungsten complex
1b resulted neither in change of the reaction course nor
in noticeable change of reaction yields.
°C (for R1 = c-C6H11).
Column chromatography purification allowed us
to isolate a single adduct. Surprisingly, the expected pyridine
ring 410 was not formed at all, but substituted 3,4-dihydropyridines
3 were obtained in high yields (Scheme 2, Table 1).
Replacement of chromium complex 1a with tungsten complex
1b resulted neither in change of the reaction course nor
in noticeable change of reaction yields.
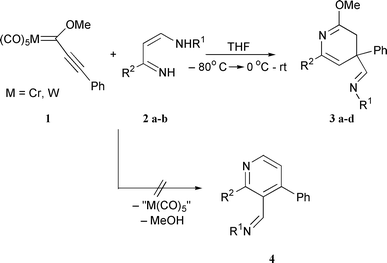 | ||
| Scheme 2 Synthesis of the 3,4-dihydropyridines 3 from alkynyl Fisher carbene complexes. | ||
The spectroscopic data found are in concordance with
structure 3. Thus, the diastereotopic hydrogen atoms attached
to the ring C3 appear as two doublets (J = 16 Hz) around δ 2.5
and 3.2 in the 1H NMR spectra. Moreover, the more characteristic
resonances in the 13C NMR spectra are found at δ 166–167 (C2), 35–36 (C3), 48–49 (C4), 104–107 (C5), 145–153 (C6) and
154–159 (CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N).
N).
The structure of compounds 3 was unambiguously confirmed by an X-ray11 diffraction experiment performed on 3d (Fig. 1).
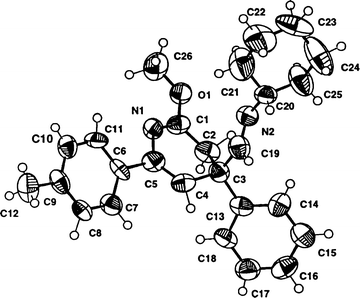 | ||
| Fig. 1 Crystal structure of the 3,4-dihydropyridine 3d. | ||
From a mechanistic point of view it is not easy to rationalize the formation of compounds 3, specifically the observed 1,2-imidoyl rearrangement. The present proposal is based primarily on the mechanism shown in Scheme 1 along with the assumption that a cyclopropane intermediate participates (Scheme 3). The reaction must be initiated by Michael-type addition of the Cβ–H enamine to form intermediate I. The second step would involve formation of the dihydropyridine intermediate II by intramolecular nitrogen addition to the metal carbene carbon. The key step is the formation of the cyclopropane species III by intramolecular 1,2-M(CO)5 migration-promoted anti nucleophilic attack at the imine function. Finally, cyclopropane ring opening of III followed by hydrogen transfer and reductive metal elimination transforms III into the dihydropyridine ring 3.
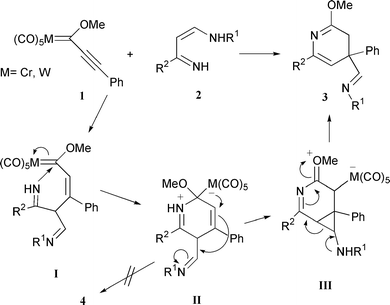 | ||
| Scheme 3 Proposed mechanism in the synthesis of the 3,4-dihydropyridines 3. | ||
In summary, we have shown that 4-amino-1-azabutadienes readily react with alkynylcarbene complexes under very mild reaction conditions affording high yields of 3,4-dihydropyri dines, whose structure is certainly unusual.12 This reaction features the following aspects: (i) a [3 + 3] N-heterocycloaddition that is rather uncommon in the field of carbene complexes, (ii) a 1,2-imidoyl shift which results in the formation of a quaternary center in preference to the expected cyclization to the pyridine ring and (iii) 1,2-metal migration.
Experimental
General methods
All reactions were carried out under a N2 atmosphere. All common reagents and solvents were obtained from commercial suppliers and used without further purification unless otherwise indicated. THF was distilled from sodium–benzophenone under a N2 atmosphere prior to use. Flash column chromatography was carried out on silica gel 60, 230–240 mesh. NMR spectra were run on a Bruker AC-300 spectrometer.Synthesis of 3,4-dihydropyridines 3a–3d
Over a 50 mL THF solution of the 4-amino-1-azabutadiene 2 (1.5 mmol) at −80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, 1.5 mmol of the alkynyl Fischer carbene complex 1 were added. The stirred solution was allowed
to reach 0
°C, 1.5 mmol of the alkynyl Fischer carbene complex 1 were added. The stirred solution was allowed
to reach 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 3a–3c and two additional days at room temperature
for 3d. Solvents were removed under vacuum
and the residue was purified by chromatographic column
over silica gel (hexane–triethylamine (10:1)).
°C for 3a–3c and two additional days at room temperature
for 3d. Solvents were removed under vacuum
and the residue was purified by chromatographic column
over silica gel (hexane–triethylamine (10:1)).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. 1H
NMR (300 MHz, CDCl3): δ 1.2–1.9 (m, 10H); 2.4 (s, 3H); 2.5 (d,
1H, J
= 15.9); 3.1 (m, 1H); 3.25 (d, 1H, J
= 15.9 Hz); 3.9 (s,
3H); 6.0 (s, 1H); 7.2–7.9 (m, 6H). 13C NMR (75 MHz, CDCl3):
δ 167.0 (s); 158.7 (d); 145.1 (s); 143.0 (s); 137.6 (s); 135.7 (s);
128.8 (d); 128.6 (d); 127.0 (d); 126.9 (d); 125.4 (d); 106.5 (d);
68.7 (d); 53.1 (q); 48.6 (s); 35.6 (t); 34.2 (t); 34.0 (t); 25.6 (t);
24.5 (t); 24.3 (t); 21.1 (q). HRMS (C26H30N2O): calculated m/z
386.23581, found 386.23558.
°C. 1H
NMR (300 MHz, CDCl3): δ 1.2–1.9 (m, 10H); 2.4 (s, 3H); 2.5 (d,
1H, J
= 15.9); 3.1 (m, 1H); 3.25 (d, 1H, J
= 15.9 Hz); 3.9 (s,
3H); 6.0 (s, 1H); 7.2–7.9 (m, 6H). 13C NMR (75 MHz, CDCl3):
δ 167.0 (s); 158.7 (d); 145.1 (s); 143.0 (s); 137.6 (s); 135.7 (s);
128.8 (d); 128.6 (d); 127.0 (d); 126.9 (d); 125.4 (d); 106.5 (d);
68.7 (d); 53.1 (q); 48.6 (s); 35.6 (t); 34.2 (t); 34.0 (t); 25.6 (t);
24.5 (t); 24.3 (t); 21.1 (q). HRMS (C26H30N2O): calculated m/z
386.23581, found 386.23558.Notes and References
- E. O. Fischer and A. Maasböl, Angew. Chem., 1964, 3, 580.
- Recent reviews: W. D. Wulff, in Comprehensi
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) e Organometallic Chemistry II, eds. E. W. Abel, F. G. A.
Stone and G. Wilkinson, Pergamon, New York, 1995, vol. 12, p. 469 Search PubMed; W. D. Wulff, Organometallics, 1998, 17, 3116 Search PubMed; J. Barluenga, Pure Appl. Chem., 1999, 71, 1385 CrossRef CAS.
e Organometallic Chemistry II, eds. E. W. Abel, F. G. A.
Stone and G. Wilkinson, Pergamon, New York, 1995, vol. 12, p. 469 Search PubMed; W. D. Wulff, Organometallics, 1998, 17, 3116 Search PubMed; J. Barluenga, Pure Appl. Chem., 1999, 71, 1385 CrossRef CAS. - R. Aumann and H. Nienabar, Ad
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) . Organomet. Chem., 1997, 41, 163 Search PubMed.
. Organomet. Chem., 1997, 41, 163 Search PubMed. - W. Chan, K. S. Chan, M. L. Yeung, R. J. Wang and T. C. W. Mak, J. Org. Chem., 1995, 60, 1741 CrossRef CAS; T. N. Danks and D. Velo-Rego, Tetrahedron Lett., 1994, 35, 9443 CrossRef CAS; J. Barluenga, M. Tomás, J. A. López-Pelegrín and E. Rubio, J. Chem. Soc., Chem. Commun., 1995, 665 RSC.
- J. Barluenga, M. Tomás, J. A. López-Pelegrín and E. Rubio, Tetrahedron Lett., 1997, 38, 3981 CrossRef CAS; R. Aumann, Z. Yu and R. Frölich, J. Organomet. Chem., 1997, 549, 311 CrossRef CAS.
- (a) J. Barluenga, M. Tomás, A. Ballesteros, J. Santamaría, R. J. Carbajo, F. López-Ortíz, S. García-Granda and P. Pertierra, Chem. Eur. J., 1996, 2, 88 CAS; (b) J. Barluenga, M. Tomás, E. Rubio, J. A. López-Pelegrín, S. García-Granda and P. Pertierra, J. Am. Chem. Soc., 1996, 118, 695 CrossRef CAS.
- R. Aumann, K. Roths and M. Grehl, Synlett, 1993, 669 CrossRef CAS.
- R. Polo, J. M. Moretó, U. Schick and S. Ricart, Organometallics, 1998, 17, 2135 CrossRef CAS.
- J. Barluenga, M. Tomás, E. Rubio, J. A. López-Pelegrín, S. García-Granda and M. Pérez, J. Am. Chem. Soc., 1999, 121, 3065 CrossRef CAS.
- The formation of this adduct would result from Michael-type addition followed by cyclization and aromatization.7.
- Crystal data for 3d: C26H30N2O, Mr = 386.52, orthorhombic, space group Pbc21, a = 6.632(2), b = 19.859(2), c = 34.049(4) Å, V = 4484(2) Å3, Z = 8, Mo-Kα radiation (graphite crystal monochromator), λ = 0.71073 Å, μ = 0.069 mm−1, T = 293(2) K. Final conventional R = 0.0591 (for 1368 FO>4σ(FO)), and wR2 = 0.2829 (for all reflections). CCDC reference number 440/215. See http://www.rsc.org/suppdata/nj/b0/b005648k/ for crystallographic files in .cif format..
- A. R. Katritzky
and C. W. Rees, Comprehensi
![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) e Heterocyclic Chemistry, eds. A. J. Boulton
and A. McKillop, Pergamon, New York, 1984. Search PubMed.
e Heterocyclic Chemistry, eds. A. J. Boulton
and A. McKillop, Pergamon, New York, 1984. Search PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2001 |
