Kinetic aspects of superfast consolidation of silicon nitride based ceramics by spark plasma sintering†
Zhijian Shen* and Mats Nygren
Department of Inorganic Chemistry, Arrhenius Laboratory, Stockholm University, S-106 91, Stockholm, Sweden. E-mail: shen@inorg.su.se; Fax: +46-8-152187; Tel: +46-8-163081
First published on UnassignedUnassigned3rd October 2000
Abstract
A newly developed novel rapid densification process named Spark
Plasma Sintering (SPS) has been successfuly applied to compact silicon
nitride based ceramics. In this process the precursor powders are pressed
uniaxially in a carbon die, and an on–off pulsed DC voltage is applied
simultaneously. The current passes through the carbon pressure die as well
as the sample, so that the sample is heated both from the outside and the
inside, which allows very fast heating rates to be applied (up to 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1).
In addition to heat and pressure this process utilises the self-heating
action caused by spark discharges between the particles, which occurs in the
initial stage of the current–voltage pulse. These processes promote
material transfer and allow the preparation of fully dense compacts of silicon
nitride based ceramics within a few minutes. In this presentation, the phase
transformation sequences during the initial stages of the sintering process,
as well as the consolidation mechanisms are discussed from a kinetic point
of view for Si3N4, β-sialon, and α–β-sialon
composite materials.
°C min−1).
In addition to heat and pressure this process utilises the self-heating
action caused by spark discharges between the particles, which occurs in the
initial stage of the current–voltage pulse. These processes promote
material transfer and allow the preparation of fully dense compacts of silicon
nitride based ceramics within a few minutes. In this presentation, the phase
transformation sequences during the initial stages of the sintering process,
as well as the consolidation mechanisms are discussed from a kinetic point
of view for Si3N4, β-sialon, and α–β-sialon
composite materials.
1. Introduction
Spark plasma sintering (SPS) is a recently developed technique that enables ceramics to be fully densified at comparatively low temperatures and in very short times. The process itself is similar to conventional hot-pressing, i.e. the powder precursors are loaded in a die made of an electrically conducting material, which is normally graphite, and a uniaxial pressure is applied during the sintering process. However instead of being heated by an external source, a direct pulsed DC current is applied, which passes through the pressure die as well as the sample, so that the sample is heated both from outside and inside. This process permits very rapid heating rates (up to 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1)
and fundamentally new sintering mechanisms. Originally, the inventors of the
spark discharge process claimed that a plasma is generated between particles
during the initial stage of the current–voltage pulse, which is why
the process was named spark plasma sintering.1
Whether or not a plasma is created has not been established yet by direct
experiment. However, it is quite obvious, from various experiments carried
out during the last five years, that the spark discharge generates internal
localised heating, which promotes material transfer and accelerates localised
reactions as well as densification.
°C min−1)
and fundamentally new sintering mechanisms. Originally, the inventors of the
spark discharge process claimed that a plasma is generated between particles
during the initial stage of the current–voltage pulse, which is why
the process was named spark plasma sintering.1
Whether or not a plasma is created has not been established yet by direct
experiment. However, it is quite obvious, from various experiments carried
out during the last five years, that the spark discharge generates internal
localised heating, which promotes material transfer and accelerates localised
reactions as well as densification.The SPS process has been applied to compacts of varous types of material. It has been shown that silicon nitride with added liquid forming components can be compacted to theoretical density by this process in a very short time (10–15 minutes).2,3 We have applied this technique in our previous work to compacts of sialon based ceramics. It was confirmed that, once it commences, the sintering finishes in a very short time. It was further noted that long-distance mass diffusion seems more restricted during this process, implying that the initial formation of sialon phases is more or less determined by the local chemistry, so one has to be aware that the obtained phase assemblages may be far from equilibrium.4 As both very high heating and cooling rates can be applied, this process provides us with a new way to follow the initial phase transformations that are otherwise very difficult to follow by conventional sintering techniques, because of the unavoidable formation of transient phases. Our work was thus expanded to include compacts of different types of silicon nitride based ceramics and their composites in an effort to understand the initial phase transformation sequences and consolidation mechanisms from a kinetic point of view, as well as to tailor the microstructure and thus to control the properties.
2. Experimental
Si3N4 (UBE, SN-E10), AlN (H.C. Starck-Berlin, grade A), Al2O3 (Alcoa, A16SG) and Yb2O3 (≥99.9%, Johnson Matthey Chemicals Ltd.) were used as powder precursors. Pure silicon nitride and β-sialon (z = 0.6), and a series of silicon nitride, β-sialon and duplex α–β-sialon ceramics, with an extra addition of Yb-doped liquid-forming material, were compacted by SPS. Their overall chemical and intended phase compositions are listed in Table 1. The obtained microstructures were compared with those previously compacted either by hot-pressing (HP) or by hot-isostatic pressing (HIP).5,6| Overall chemical compositionb | ||||||
|---|---|---|---|---|---|---|
| Sample | Intended phase compositiona | Yb2O3 | Al2O3 | AlN | Si3N4 | SiO2 |
| a Remarks: α-sialon = YbxSi12 − 4.5xAl4.5xO1.5xN16 − 5x; β = β-sialon, Si6 − zAlzOzN8 − z; Overall composition of the extra liquid-forming materials: L1 = Yb2Si2O7, L2 = Yb0.25Al0.5Si0.25O1.30N0.217, respectively.b The chemical compositions are expressed in wt%. | ||||||
| a | α-Si3N4 | — | — | — | 100 | — |
| b | β (z = 0.6) | — | 4.33 | 5.29 | 90.41 | — |
| c | β-Si3N4 + 10 vol% L1 | 13.21 | — | — | 85.14 | 1.65 |
| d | β(z = 0.6) + 10 vol% L2 | 7.50 | 8.49 | 3.89 | 80.12 | — |
| e | α/β (1∶1) + 10 vol% L2 | 12.55 | 6.44 | 6.87 | 74.14 | — |
The SPS processing was carried out under vacuum in a spark plasma sintering
apparatus, Dr. Sinter 1050 (Sumitomo Coal Mining Co. Ltd., Japan).
The powder precursors were loaded in a cylindrical carbon die with an inner
diameter of 20 mm. The sintering temperature was set to 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C,
the heating rate to 200
°C,
the heating rate to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1, and the
holding time to 0 or 5 minutes. A pressure of 50 MPa was applied
from the start to the end of the dwell time. The shrinkage and shrinkage rate
during the sintering process were recorded, and the obtained data were corrected
for the thermal expansion of the graphite punches. The set-up allows a
cooling rate of 350
°C min−1, and the
holding time to 0 or 5 minutes. A pressure of 50 MPa was applied
from the start to the end of the dwell time. The shrinkage and shrinkage rate
during the sintering process were recorded, and the obtained data were corrected
for the thermal expansion of the graphite punches. The set-up allows a
cooling rate of 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 in the temperature
range: 1700–1100
°C min−1 in the temperature
range: 1700–1100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
°C.
The microstructures were characterised by scanning electron microscopy (SEM) studies on carefully polished surfaces, using a JEOL 880 instrument equipped with an energy-dispersive X-ray spectroscopy (EDS) microanalysis facility (Link ISIS). Crystalline phases were determined from X-ray powder diffraction patterns obtained in a Guinier–Hägg camera, using Cu-Kα1 radiation with Si as internal standard. The unit cell dimensions of the formed phases were refined by the PIRUM program,7 from which the z-values of the β-sialon phases were calculated by applying the equations given by Ekström et al.8 The phases present, their α/(α + β) ratio, and the unit cell dimensions of either silicon nitride or the various sialon phases formed in all samples are listed in Table 2.
| α-phase unit cell | β-phase unit cell | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Sintering conditions | Phase assemblies | α/(α + β) (%) | a/Å | c/Å | a/Å | c/Å | z-value |
| a Remarks: α = α-Si3N4; β = β-Si3N4; α′ = α-sialon; β′ = β-sialon, G = a glass phase. | ||||||||
| a | SPS 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/0 min °C/0 min | α + β | 90 | 7.755 | 5.620 | 7.605 | 2.907 | 0 |
| b | SPS 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/0 min °C/0 min | β′ + α | 10 | 7.755 | 5.620 | 7.619 | 2.919 | 0.49 |
SPS 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/5 min °C/5 min | β′ | 0 | 7.620 | 2.920 | 0.55 | |||
HIP 1820![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/1 h, 200 MPa °C/1 h, 200 MPa | β′ | 0 | 7.618 | 2.919 | 0.50 | |||
| c | SPS 1600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/0 min °C/0 min | α + β + G | 59 | 7.755 | 5.620 | 7.605 | 2.907 | 0 |
SPS 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/0 min °C/0 min | β + α + G | 43 | 7.755 | 5.620 | 7.605 | 2.907 | 0 | |
SPS 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/5 min °C/5 min | β + G | 0 | 7.605 | 2.908 | 0 | |||
HP 1750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/2 h, 30 MPa °C/2 h, 30 MPa | β + α + G + Yb2Si2O7 | 8 | 7.753 | 5.621 | 7.606 | 2.908 | 0 | |
| d | SPS 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/0 min °C/0 min | β′ + G | 0 | 7.625 | 2.925 | 0.71 | ||
HP 1750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/2 h, 30 MPa °C/2 h, 30 MPa | β′ + G | 0 | 7.629 | 2.924 | 0.77 | |||
| e | SPS 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/0 min °C/0 min | β′ + α′ + G | 32 | 7.803 | 5.671 | 7.631 | 2.927 | 0.85 |
HP 1750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C/2 h, 30 MPa °C/2 h, 30 MPa | β′ + α′ + G | 32 | 7.803 | 5.683 | 7.629 | 2.926 | 0.80 | |
3. Results and discussion
Fig. 1 shows the shrinkage curves, which reveal that no shrinkage takes place in the pure silicon nitride sample even up to 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Once it does take place the shrinkage occurs
very rapidly in all the other samples. The pure silicon nitride sample after
compacting at 1700
°C. Once it does take place the shrinkage occurs
very rapidly in all the other samples. The pure silicon nitride sample after
compacting at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C is still very porous and easy to crush, and
retains its original phase composition, i.e. the α/(α + β)
ratio remains at 90% after compacting. This result indicates that although
material transfer may be promoted by the SPS process, the presence of a liquid
is necessary to compact a mainly covalently bonded compound such as Si3N4,
by facilitating the α–β transformation and enhancing densification via
a solution–precipitation mechanism.
°C is still very porous and easy to crush, and
retains its original phase composition, i.e. the α/(α + β)
ratio remains at 90% after compacting. This result indicates that although
material transfer may be promoted by the SPS process, the presence of a liquid
is necessary to compact a mainly covalently bonded compound such as Si3N4,
by facilitating the α–β transformation and enhancing densification via
a solution–precipitation mechanism. | ||
Fig. 1 Shrinkage curves recorded
during SPS sintering at a rate of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1
up to 1700 °C min−1
up to 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. The curves are labelled in the same way as in Table 1. °C. The curves are labelled in the same way as in Table 1. | ||
The pure β-sialon (z = 0.6)
sample shows a dramatic shrinkage in the temperature interval: 1400–1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
Thereafter the shrinkage continues at a lower rate until the final sintering
temperature, 1700
°C.
Thereafter the shrinkage continues at a lower rate until the final sintering
temperature, 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. With no dwell time at 1700
°C. With no dwell time at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C,
the sample gives a density value of 2.98 g cm−1,
corresponding to 95% of the theoretical density. The final phase composition,
as revealed by the XRD patterns shown in Fig. 2,
indicates that the α–β transformation does not go to completion
with such a short sintering duration (1.5 minutes from 1400 to
1700
°C,
the sample gives a density value of 2.98 g cm−1,
corresponding to 95% of the theoretical density. The final phase composition,
as revealed by the XRD patterns shown in Fig. 2,
indicates that the α–β transformation does not go to completion
with such a short sintering duration (1.5 minutes from 1400 to
1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C). The sample is, however, already more dense than
the one compacted by HIP at 1820
°C). The sample is, however, already more dense than
the one compacted by HIP at 1820![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, where the α–β
transformation is complete after a 1 hour hold but the density is only
87% of the theoretical density. With a dwell time of 5 minutes
at 1700
°C, where the α–β
transformation is complete after a 1 hour hold but the density is only
87% of the theoretical density. With a dwell time of 5 minutes
at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, a fully compacted body was obtained by SPS with no
noticeable residual α-Si3N4, implying both
complete transformation and complete densification.
°C, a fully compacted body was obtained by SPS with no
noticeable residual α-Si3N4, implying both
complete transformation and complete densification.
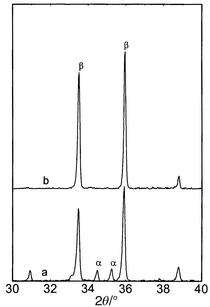 | ||
Fig. 2 The X-ray powder
diffraction patterns of the pure β-sialon (z = 0.6)
SPSed at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C with no (a) and 5 minutes (b)
holding time. °C with no (a) and 5 minutes (b)
holding time. | ||
For the other three samples the shrinkage stops before 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C,
at 1670, 1580, and 1450
°C,
at 1670, 1580, and 1450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for sample c, d and e respectively,
indicating that all of these samples were fully densified by 1700
°C for sample c, d and e respectively,
indicating that all of these samples were fully densified by 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
XRD analysis reveals, however, that the α–β transformation
is not complete in sample c after sintering at 1700
°C.
XRD analysis reveals, however, that the α–β transformation
is not complete in sample c after sintering at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C without
a hold. Approximately 43% of the Si3N4 remains
as α-phase, but this completely transforms to β-phase after
holding for only 5 minutes at 1700
°C without
a hold. Approximately 43% of the Si3N4 remains
as α-phase, but this completely transforms to β-phase after
holding for only 5 minutes at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, see Fig. 3
and Table 2. For comparison, a sample
from the same batch compacted at 1700
°C, see Fig. 3
and Table 2. For comparison, a sample
from the same batch compacted at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 2 hours by
hot-pressing still contains 8% residual α-Si3N4
phase. Despite a smaller grain size, the phase composition and microstructure
of samples with overall compositions of d and e, SPSed at 1700
°C for 2 hours by
hot-pressing still contains 8% residual α-Si3N4
phase. Despite a smaller grain size, the phase composition and microstructure
of samples with overall compositions of d and e, SPSed at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
with no holding time, mimic to a large extent those obtained after hot-pressing
at 1750
°C
with no holding time, mimic to a large extent those obtained after hot-pressing
at 1750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 2 hours, see Figs. 4
and 5.
°C for 2 hours, see Figs. 4
and 5.
 | ||
Fig. 3 The X-ray powder
diffraction patterns of the silicon nitride with 10 vol% extra
liquid forming material SPSed at 1600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C with no holding (a)
and at 1700 °C with no holding (a)
and at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C with no (b) and 5 minutes (c)
holding time. °C with no (b) and 5 minutes (c)
holding time. | ||
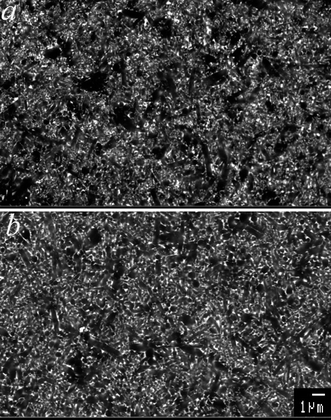 | ||
Fig. 4 Back-scattered
electron scanning micrographs showing the microstructures of the β-sialon
with 10 vol% extra liquid forming material SPSed at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
with no holding (a) and hot-pressed at 1750 °C
with no holding (a) and hot-pressed at 1750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for
2 hours (b). Needle-like grains are β-sialon,
white spots are due to the glass phase. °C for
2 hours (b). Needle-like grains are β-sialon,
white spots are due to the glass phase. | ||
 | ||
Fig. 5 Back-scattered
electron scanning micrographs showing the microstructures of the α-β-sialon
with 10 vol% extra liquid forming material SPSed at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
with no holding (a) and hot-pressed at 1750 °C
with no holding (a) and hot-pressed at 1750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for
2 hours (b). β-Sialon, α-sialon and glass
phase have black, grey and white contrast, respectively. °C for
2 hours (b). β-Sialon, α-sialon and glass
phase have black, grey and white contrast, respectively. | ||
In Fig. 1, a shoulder can be seen
on the shrinkage curves of samples c, d and e before the main shrinkage takes
place. This most probably indicates that a eutectic oxide liquid phase has
already formed at 1250–1450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C in sample c, and 1250–1350
°C in sample c, and 1250–1350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
in samples d and e. It is reasonable to assume that more liquid forms when
the precursor nitride powders start to dissolve with increasing temperature.
Although the amount of liquid formed in these samples is designed to be similar, i.e.,
10% by volume, the properties of the liquid phase that forms may vary
with the overall compostion of the starting powder mixtures. The liquid that
forms in sample c is thus expected to be more viscous than those that form
in samples d and e, because when shifting the liquid composition from the
Yb–Si–O–N to the Yb–Al–Si–O–N system,
both the eutectic temperature and the viscosity of the liquid are reduced.
This fact may explain why samples d and e can be compacted at a comparatively
much lower temperature.
°C
in samples d and e. It is reasonable to assume that more liquid forms when
the precursor nitride powders start to dissolve with increasing temperature.
Although the amount of liquid formed in these samples is designed to be similar, i.e.,
10% by volume, the properties of the liquid phase that forms may vary
with the overall compostion of the starting powder mixtures. The liquid that
forms in sample c is thus expected to be more viscous than those that form
in samples d and e, because when shifting the liquid composition from the
Yb–Si–O–N to the Yb–Al–Si–O–N system,
both the eutectic temperature and the viscosity of the liquid are reduced.
This fact may explain why samples d and e can be compacted at a comparatively
much lower temperature.
Once a liquid phase is formed, densification progresses very fast in the
SPS process. In the simplest case of Si3N4 doped with
10 vol% liquid-forming material (sample c), the
sample is already fully densified at 1600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, although at this
stage the α–β transformation is far from complete, and the
liquid phase is not distributed evenly through the sample due to the lack
of diffusion, see the SEM picture shown in Fig. 6.
It can also be noticed in this figure that the grain growth is tremendously
fast. Thus, the average size for sample c compacted by SPS at 1700
°C, although at this
stage the α–β transformation is far from complete, and the
liquid phase is not distributed evenly through the sample due to the lack
of diffusion, see the SEM picture shown in Fig. 6.
It can also be noticed in this figure that the grain growth is tremendously
fast. Thus, the average size for sample c compacted by SPS at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
for 5 minutes is even larger than for the sample from the same batch
compacted by hot-pressing at 1750
°C
for 5 minutes is even larger than for the sample from the same batch
compacted by hot-pressing at 1750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 2 hours.5 This seems to indicate that the grain-coarsening
in SPSed samples is more likely to be controlled by interface reactions, rather
than by diffusion.
°C for 2 hours.5 This seems to indicate that the grain-coarsening
in SPSed samples is more likely to be controlled by interface reactions, rather
than by diffusion.
 | ||
Fig. 6 Back-scattered
electron scanning micrographs showing the microstructures of the silicon nitride
with 10 vol% extra liquid forming material SPSed at 1600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
with no holding (a) and at 1700 °C
with no holding (a) and at 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C with no (b)
and 5 minutes (c) holding time. °C with no (b)
and 5 minutes (c) holding time. | ||
4. Conclusions
In summary, the present work shows that various types of silicon nitride based ceramics can be compacted to full density by the SPS process at lower temperatures and with substantially shorter holding times than required for hot pressing or HIP. It is confirmed that the formation of a liquid phase is necessary to facilitate the α–β transformation and enhance densification via a solution–precipitation mechanism. The phase assemblages in fully compacted bodies, however, may be far from equilibrium due to the very fast consolidation. This opens up new possibilities to tailor the microstructure; the α/(α + β) ratio, grain size, as well as grain morphology can be changed by simple variation of the SPS sintering parameters. On the other hand, one has to be aware that some SPSed materials are metastable, implying that the materials may later approach equilibrium, changing their microstructure and properties, especially during service at higher temperatures. The grains grow so fast during the SPS process, that diffusion-controlled grain growth mechanisms proposed for traditional liquid-sintering seems not fit to the present observations. The grain-coarsening in SPSed samples seems to be controlled more by interface reactions.References
- M. Tokita, Nyu Seramikkusu, 1997, 10, 43 Search PubMed.
- T. Nishimura, M. Mitomo, H. Hirotsuru and M. Kawahara, J. Mater. Sci. Lett., 1995, 14, 1046 CrossRef CAS.
- D. S. Perera, M. Tokita and S. Moricca, J. Eur. Ceram. Soc., 1998, 18, 401 CrossRef CAS.
- Z. Shen and M. NygrenJ. Eur. Ceram. Soc., 2000, in press. Search PubMed.
- Z. Shen and M. Nygren, in 6th International Symposium on Ceramic Materials & Components for Engines, ed. K. Niihara, S. Hirano, S. Kanzaki, K. Komeya and K. Morinaga, Japan, 1997, p. 627–631. Search PubMed.
- P. Pettersson, Z. Shen, M. Johnsson and M. Nygren, J. Am. Ceram. Soc., 2000, in press. Search PubMed.
- P. E. Werner, Ark. Kemi, 1969, 31, 513 Search PubMed.
- T. Ekström, P. O. Käll, M. Nygren and P. O. Olsson, J. Mater. Sci., 1989, 24, 1853.
Footnote |
| † Basis of a presentation given at Materials Discussion No. 3, 26–29 September 2000, University of Cambridge, UK. |
| This journal is © The Royal Society of Chemistry 2001 |
