Electrical properties of the oxygen-deficient perovskites, Ca2Mn2 − xNbxOγ: 0 ≤ x ≤ 1.2, with Mn valence varying from +2.0 to +4.0†
Angela
Kruth
and
Anthony
R. West
Department of Engineering Materials, University of Sheffield, Mappin Street, Sheffield, UK S1 3JD
Abstract
The electrical conductivity of a range of ceramics of general formula Ca2Mn2 − xNbxOγ, with x and γ as variables, was measured using impedance spectroscopy. All materials were semiconducting but conductivity decreased with both increasing x and decreasing γ, both of which involved a gradual decrease in oxidation state of Mn from +4 in CaMnO3 to +2 in Ca2Mn0.8Nb1.2O5.8. Possible factors that control the conductivity are discussed; electrons in eg orbitals of Mn appear not to be involved directly; instead, conduction appears to depend either on the degree of t2g–Opπ–t2g orbital overlap or on σ d–p overlap, with donation of electrons from oxygen into the bonds involving eg orbitals. In both cases, the conductivity is influenced mainly by Mn–O–Mn distances and unit cell dimensions. For compositions of intermediate oxygen content γ, it was difficult to obtain materials that were electrically homogeneous due to oxygen content gradients; thus, samples prepared by partial reduction in H2 were more heavily reduced at the grain surfaces than in the grain interiors. Since the conductivity was very sensitive to γ, impedance spectroscopy provided a valuable method to investigate oxygen inhomogeneity. Two types of sample inhomogeneity were characterized: those with reduced surfaces and oxidized cores and vice versa, those with oxidized surfaces and reduced cores.
Introduction
The perovskite structure is able to tolerate great variations in its stoichiometry, especially oxygen content. Oxygen deficiency has attracted much attention because of its association with a range of phenomena, such as mixed electronic–ionic conduction, superconductivity, giant magnetoresistance and catalytic activity. In perovskites which contain transition metals, especially Cr, Mn, Fe, Co and Ni, the existence of mixed or intermediate valence states is critical for those properties.1–5The Ca2Mn2 − xNbxOγ system: 0 ≤ x ≤ 1.2, can accommodate up to 20% vacancies at oxygen sites and Mn occurs in valence states ranging from +2 to +4.6 Mn and Nb are disordered over the B-sites, at least for composition x = 1.0.7 Depending on the average B-cation composition and oxygen content, two solid solutions form: an extensive orthorhombic GdFeO3-type solid solution over the range 0 ≤ x ≤ 1.2 with zero or small oxygen deficiency, and a closely-related, grossly oxygen-deficient solid solution over the range 0.3 ≤ x ≤ 0.8 with a simple cubic perovskite structure. The unit cell volume, V, and the orthorhombic distortion of the perovskite unit cell due to tilting of MnO6 octahedra vary with x, γ and Mn valency, as follows: (i) with increasing x (and decreasing Mn valency) at γ = 6, V and the orthorhombic distortion increase; (ii) on removal of oxygen (and decrease in Mn valency), V increases but the orthorhombic distortion decreases.
Several factors influence the electronic conductivity of transition metal oxides. For the present mixed valence Mn oxides, these include: Mn valence state, Nb content, unit cell size and Mn–O–Mn bond angles. Taguchi8 studied CaMnO3 − δ with Mn valence ranging from +3.7 to +3.94 and found the conductivity to depend on the oxygen content; on reduction, σ increased, suggesting an n-type conduction mechanism. By contrast, the conductivity of composition x = 1.0, with Mn valence in the range +2.8 to +3.0, decreased on reduction; however, oxygen loss occurred preferentially from surface and grain boundary regions leading to oxygen concentration gradients through the samples.
In order to understand which parameters control the conductivity of Ca2Mn2 − xNbxOγ, a number of fully and partially oxidised samples with x = 0.5 and 1.2 have been studied and the results compared with those for x = 0.0 and 1.0. For x = 0.5, γ varies over the range 5.0 ≤ γ ≤ 6.0 and the average Mn valence varies from +2.3 to +3.7; the structure changes from orthorhombic GdFeO3-type to cubic perovskite type with decreasing γ. For x = 1.2, the orthorhombic GdFeO3 structure is retained over the range 5.8 ≤ γ ≤ 6.0 and the Mn valence varies from +2.0 to +2.5. On reoxidation, Ca2Mn0.8Nb1.2O5.8 forms a poorly characterized, oxygen-rich phase of nominal composition Ca2Mn0.8Nb1.2O6.2 with Mn valence +3.0; this loses the oxygen excess at higher temperatures and reforms single phase Ca2Mn0.8Nb1.2O6.0.
Experimental
Compositions Ca2Mn2 − xNbxOγ were prepared, as previously,6,7 from CaCO3, MnO2 and Nb2O5, which were mixed and fired, first at 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and then at 1250 to 1350
°C and then at 1250 to 1350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C in steps of 50
°C in steps of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C with intermediate regrinding. Products were heated finally at 1400
°C with intermediate regrinding. Products were heated finally at 1400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 3 days and quenched in air. Pellets of 3–8 mm diameter and 1–2 mm thickness were cold-pressed at ∼200 MPa, sintered overnight at 1400
°C for 3 days and quenched in air. Pellets of 3–8 mm diameter and 1–2 mm thickness were cold-pressed at ∼200 MPa, sintered overnight at 1400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and quenched in air; typical pellet densities were 70–80% of theoretical.
°C and quenched in air; typical pellet densities were 70–80% of theoretical.
In order to vary the oxygen content, pellets were post-reaction heat-treated in O2, N2 and 10% H2/90% N2. In high pressure O2, pellets were heated at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 to 800
°C min−1 to 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, held for 16 h and cooled at 2
°C, held for 16 h and cooled at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 in a Morris Research Furnace, HPS-5015E7. The maximum pressure was ∼100 atm at 800
°C min−1 in a Morris Research Furnace, HPS-5015E7. The maximum pressure was ∼100 atm at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. In flowing N2, pellets were heated at 10
°C. In flowing N2, pellets were heated at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 to 1100
°C min−1 to 1100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, held for 10 h and cooled at 2
°C, held for 10 h and cooled at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1. Heat treatments in H2/N2 were carried out in a Stanton Redcroft TG-DTA 1500 which allowed the weight losses to be determined in situ. Samples were heated at 10
°C min−1. Heat treatments in H2/N2 were carried out in a Stanton Redcroft TG-DTA 1500 which allowed the weight losses to be determined in situ. Samples were heated at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 to temperature, cooled at 20
°C min−1 to temperature, cooled at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 to ∼350
°C min−1 to ∼350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and more slowly thereafter to room temperature. The oxygen partial pressure in these experiments was not buffered since the TG furnace was not leak-proof. The results cannot be related to a particular oxygen partial pressure, therefore, and partially-reduced samples in particular may not have reached a homogeneous, equilibrium state; there was evidence in some cases that grain boundaries were more heavily reduced than grain cores, giving rise to oxygen concentration gradients. Nevertheless, this method was effective as a means of varying the oxygen content and average Mn oxidation state.
°C and more slowly thereafter to room temperature. The oxygen partial pressure in these experiments was not buffered since the TG furnace was not leak-proof. The results cannot be related to a particular oxygen partial pressure, therefore, and partially-reduced samples in particular may not have reached a homogeneous, equilibrium state; there was evidence in some cases that grain boundaries were more heavily reduced than grain cores, giving rise to oxygen concentration gradients. Nevertheless, this method was effective as a means of varying the oxygen content and average Mn oxidation state.
Oxygen contents of various pellets were determined from the weight loss during complete reduction of Mn3+ and Mn4+ to Mn2+ in 10% H2/90% N2. Pellets were heated at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 until the weight remained constant; it was assumed then that all Mn was reduced to Mn2+. From the total weight loss, the initial oxygen content was calculated. Phase identity and purity were determined by X-ray diffraction, using a Hägg–Guinier camera and CuKα1 radiation.
°C min−1 until the weight remained constant; it was assumed then that all Mn was reduced to Mn2+. From the total weight loss, the initial oxygen content was calculated. Phase identity and purity were determined by X-ray diffraction, using a Hägg–Guinier camera and CuKα1 radiation.
For impedance measurements, organo-Ag paste electrodes were attached to opposite pellet faces and dried at 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 1 h in order to remove organic residue. Thin gold strips were held to the sample by pressure. For measurements over the range 298–793 K, the conductivity jig was inserted in a vertical tube furnace; for measurements at 200–300 K, the jig was fixed inside a Dewar flask containing a dry-ice/acetone liquid bath. An Oxford Optistat Cryostat with an IT C4 temperature controller was employed for data collection at T ≥ 83 K. Measurements in the furnace and dry-ice/acetone were carried out in air; those in the cryostat used N2. Data were collected over the frequency range 10−2 to 107 Hz using Solartron 1250/1287 and Hewlett-Packard 4192A LF Impedance Analysers. All data were corrected for sample geometry.
°C for 1 h in order to remove organic residue. Thin gold strips were held to the sample by pressure. For measurements over the range 298–793 K, the conductivity jig was inserted in a vertical tube furnace; for measurements at 200–300 K, the jig was fixed inside a Dewar flask containing a dry-ice/acetone liquid bath. An Oxford Optistat Cryostat with an IT C4 temperature controller was employed for data collection at T ≥ 83 K. Measurements in the furnace and dry-ice/acetone were carried out in air; those in the cryostat used N2. Data were collected over the frequency range 10−2 to 107 Hz using Solartron 1250/1287 and Hewlett-Packard 4192A LF Impedance Analysers. All data were corrected for sample geometry.
At this point, it is necessary to comment on the correction of electrical data for sample geometry. Conductivities are calculated from resistance data using:
 | (1) |
Results
Composition x = 0.5
Electrical characterisation of composition x = 0.5 was carried out on four pellets: as-prepared (quenched in air from 1400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C) and after post-reaction heat-treatment in O2, N2 and 10% H2/90% N2. In H2/N2, it was more difficult to reduce a pellet than a finely powdered sample; the pellet had to be heated to higher temperature in order to obtain the same oxygen content and took longer to equilibrate at a given temperature. It is shown later that the oxygen content may vary significantly throughout a pellet, making it difficult to obtain homogeneous samples during reduction. From TG, the as-prepared pellet and pellets annealed in O2 and N2 all had oxygen contents, γ, close to 6.0 and Mn in valence state ∼+3.7. The pellet heated in H2/N2 had γ ∼ 5.34(2) and Mn valence state +2.8.
°C) and after post-reaction heat-treatment in O2, N2 and 10% H2/90% N2. In H2/N2, it was more difficult to reduce a pellet than a finely powdered sample; the pellet had to be heated to higher temperature in order to obtain the same oxygen content and took longer to equilibrate at a given temperature. It is shown later that the oxygen content may vary significantly throughout a pellet, making it difficult to obtain homogeneous samples during reduction. From TG, the as-prepared pellet and pellets annealed in O2 and N2 all had oxygen contents, γ, close to 6.0 and Mn in valence state ∼+3.7. The pellet heated in H2/N2 had γ ∼ 5.34(2) and Mn valence state +2.8.
The three pellets with γ = 6.0 were highly conducting at room temperature and, therefore, were measured at low temperatures in the cryostat. Fig. 1 shows the impedance complex plane plot for the as-prepared pellet at 83 K. The total resistance was obtained from the intercept with the Z′ axis and was Rtotal ∼ 10.5 kΩ cm. Note that for these and all subsequent plots, the R and C data were corrected for pellet geometry. Three responses were partially resolved in the plot. The initial assumption was made that these data can be represented by an equivalent circuit composed, ideally, of three parallel RC elements in series, Fig. 1 inset. The low frequency response was assigned to element R1C1, the intermediate frequency response to R2C2 and the high frequency response to R3C3. Arcs, attributed to R2C2 and R3C3, were poorly resolved and, therefore, R2 and R3 values (Table 1) were obtained only approximately; however, R3 is larger than R2 and R1, indicating that R3C3 is the most resistive element in this sample at this temperature. From the Z″, M″ spectroscopic plots of the same data, Fig. 2, the high frequency peak had capacitance C3 ∼ 9 pF, calculated from the relation
 | (2) |
 | ||
Fig. 1
Composition x = 0.5, heated in air at 1400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C: impedance complex plane plot at 83 K. Inset: equivalent circuit representing three parallel RC elements in series. °C: impedance complex plane plot at 83 K. Inset: equivalent circuit representing three parallel RC elements in series.
| ||
 | ||
Fig. 2
Composition x = 0.5, heated in air at 1400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C: combined spectroscopic Z′′, ○, M′′, ●, plot at 83 K; same data as used in Fig. 1. °C: combined spectroscopic Z′′, ○, M′′, ●, plot at 83 K; same data as used in Fig. 1.
| ||
| O2 (83 K) | air (83 K) | N2 (83 K) | H2/N2 (250 K) | H2/N2 (170 K) | |
|---|---|---|---|---|---|
| a From M″ data. | |||||
| R total/kΩ | 11.5 | 10.5 | 21.7 | 185 | |
| R 1/kΩ | 2.0(3) | 2.6(1) | 7.9(7) | 10(3) | |
| R 2/kΩ | 2.9(3) | 2.2(1) | 8.0(7) | 35(6) | |
| R 3/kΩ | 6.6(3) | 5.7(3) | 5.8(7) | 140(6) | 8100(400) |
| R 4/kΩ | 20(10)a | ||||
| C 1/nF cm−1 | 8(4) | 15(6) | 5(2) | 200(50) | |
| C 2/nF cm−1 | 0.7(2) | 1.1(5) | 0.05(2) | 1.4(4) | |
| C 3/pF cm−1 | 10(3) | 9(3) | 14(4) | 9(2) | 16(4) |
| C 4/pF cm−1 | 4 | 5(1)a | |||
If R1C1 were associated with a non-ohmic electrode contact, the associated resistance should increase as the applied voltage is decreased. In Fig. 3, a decrease in amplitude of the ac voltage from 1 V to 100 mV led to a significant increase of R1; R2 and R3 did not vary. When Au paste or In–Ga eutectic were applied as electrodes, similar effects were seen but with an even higher value for R1. Therefore, R1C1 represents a non-ohmic contact at the electrode/sample interface. All subsequent measurements were carried out using Ag electrodes and an ac voltage of 1 V. This reduced the magnitude of R1 but did not cause any changes to R2 and R3.
 | ||
Fig. 3
Composition x = 0.5, heated in air at 1400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C: data at 83 K for applied ac voltages of 100 mV, ●, and 1 V, ○. °C: data at 83 K for applied ac voltages of 100 mV, ●, and 1 V, ○.
| ||
Impedance plots at 83 K for pellets annealed in O2 and N2 were broadly similar to those of the as-prepared sample. For the pellet treated in O2, R values were similar, Table 1. For the pellet treated in N2, R3 changed little but R1 and R2 increased significantly and had values similar to R3. C1 and C3 showed little variation after heat treatment in O2 and N2, whereas C2, assigned to the grain boundary region, varied from 0.03–0.07 nF in N2 to 0.5–0.9 nF in O2 and 0.6–1.6 nF in air, indicating that the grain boundary layers were relatively thin after treatment in air and O2, but became thicker in N2.‡ This appears to be because the grain boundary regions are oxygen-deficient and increase in thickness, due to a small amount of O2 loss, on heating in N2.
The fourth pellet, with γ = 5.34, was much more resistive, Table 1. C3 was similar to the bulk capacitance of oxidised compositions; C1 and C2 were larger but from their magnitudes can still be attributed to an electrode/sample interface and a grain boundary, respectively. M″ data, Fig. 4, show a main peak at 3 MHz and a poorly resolved shoulder peak at 200 kHz. The shoulder occurs at a similar position to the main peak of the Z″ spectrum and was attributed to element R3C3. The M″ maximum at 3 MHz was assigned to a second bulk element, R4C4. Bulk M″ peaks were better separated at lower temperatures which indicates that R3 has a greater temperature dependence and therefore higher activation energy than R4 as shown by Arrhenius plots of fmax for the Z″ (R3C3) and M″ (R4C4) peaks, Fig. 5. If it is assumed that C3 and C4 are independent of temperature, then the activation energies, Ea, for fmax correspond to those for the respective R values. Ea for R4C4 (0.13 eV) is smaller than for R3C3 (0.21 eV) and higher than for R3C3 in fully oxidised samples (0.09 eV, see later). Values of R4 and C4 calculated from M″ data are shown in Table 1. R4 is much smaller than R3 and R3C3 is therefore the more resistive bulk region. From the observed dependence of resistance on oxygen content, it appears that R3C3 corresponds to the outer grain region which is more reduced than the inner R4C4 grain core. The value of C4 is smaller than that of C3; consequently, the reduced, outer region is thinner than the less-reduced core.
 | ||
Fig. 4
Composition x = 0.5, heat-treated in H2/N2 at 580![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C: combined spectroscopic M′′, Z′′ plot at 250 K. °C: combined spectroscopic M′′, Z′′ plot at 250 K.
| ||
 | ||
| Fig. 5 Arrhenius plots of log fmax for bulk responses, R3C3 and R4C4, of composition x = 0.5, heat-treated in air, ●, O2, □, N2, ▽, and H2/N2; R3C3 (white circles) and R4C4 (grey circles). Average Mn valence states, +3.7 and +2.8, for different data sets are indicated. | ||
Bulk and grain boundary conductivities, σ3 and σ2, of all four samples are plotted in Arrhenius format in Figs. 6a and b: note that the σ2 data refer to the overall geometry of the pellet and not to that of the grain boundary region. Samples annealed in air, O2 and N2 with Mn average valence state +3.7 had similar bulk conductivities and Ea, ∼0.09 eV. After reduction in H2/N2 to give Mn average valence state +2.8, σ3 decreased drastically and Ea increased to ∼0.23 eV although the Arrhenius plot may be slightly curved. The variation in activation energies between oxidised and reduced samples may either indicate the existence of distinct conduction mechanisms in samples with different Mn average valencies, or that the difficulty of electron hopping between adjacent Mn atoms is composition-dependent. This is discussed later (Fig. 11) where it is concluded that a single conduction mechanism occurs.
 | ||
| Fig. 6 Arrhenius plots of (a) bulk and (b) grain boundary conductivities, σ3 and σ2, of composition x = 0.5, heat treated in air, ●, O2, □, N2, ▽ and H2/N2, ○. | ||
Grain boundary conductivities, σ2, Fig. 6b, are similar for samples heated in air and O2, but decrease significantly in N2 and greatly in H2/N2. Ea values are similar for samples heated in air, O2 and N2 (∼0.09 eV), but much larger in H2/N2. Since activation energies for bulk and grain boundary regions in air, O2 and N2 are similar, 0.09 eV, the conduction mechanism is the same in both regions.
A schematic model for conduction through partially reduced grains is shown in Fig. 7a. Grain shells (R3C3) are more resistive than cores (R4C4) and where possible, charge carriers travel through the inner bulk regions. It is shown later that this is not always the case and the conduction path may vary greatly depending on the thermal history. XRD of the reduced pellet showed a cubic pattern, similar to those for oxygen-deficient samples with x = 0.5.7 However, the lines were diffuse, probably due to variation of γ within the sample bulk.
 | ||
| Fig. 7 Conduction pathways through inhomogeneous grains: (a) partially reduced grain after heat-treatment of a fully oxidised sample in H2/N2; (b) partially re-oxidised grain after heating of a reduced composition in air. | ||
Composition x = 1.2
In Nb-rich compositions, the oxygen content varies over a smaller range than in Mn-rich compositions.7 Two pellets were prepared: the as-prepared pellet had γ = 6.0 and Mn valence state +2.5; the other pellet was reduced in H2/N2, had γ = 5.8 and all Mn reduced to Mn2+. Note that +2.5 is not the highest average valence state of Mn which can be achieved for this cation composition; on heating the reduced sample in air, the average Mn oxidation state increases to +3.0 to yield a metastable phase of composition Ca2Mn0.8Nb1.2O6.2. This excess oxidation is important for the interpretation of impedance data, as seen later.The −Z″, Z′ plane for the as-prepared pellet at 278 K showed a large arc with R ∼ 3.9 MΩ cm and C ∼ 2(3) pF, which was assigned to the bulk region, R3C3. A second, small arc at low f had ∼10 nF, and was attributed to either the grain boundary, R2C2, or the electrode/sample interface, R1C1; it was not investigated further.
The reduced pellet with γ = 5.8 was the most resistive sample investigated. The −Z″, Z′ plane (not shown) had a single arc with R3 ∼ 9.4 MΩ cm and C ∼ 5–9 pF at 508 K, Table 2. M″ data (not shown) showed a single, symmetric peak attributed to R3C3; in this case, the sample bulk appeared not to separate into regions of different oxygen content on reduction.
| Air (278 K) | Air (472 K) | H2/N2 (508 K) | |
|---|---|---|---|
| R 1,2/kΩ | 2.6(1) | ||
| R 3/kΩ | 3900(300) | 4.8(3) | 9400(400) |
| C 1,2/nF cm−1 | 10(2) | ||
| C 3/pF cm−1 | 2(3) | 7(2) |
C
3 showed little variation with temperature and was 2–7 pF for as-prepared and reduced pellets. Bulk conductivities, σ3, are given in Arrhenius format in Fig. 8. For γ = 6.0, data, *, fell on a straight line with Ea ∼ 0.39 eV and were reversible on cooling. Data for γ = 5.8, however, showed more complex behaviour, attributed to partial reoxidation of the sample ≥400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. On first heating, ●, data fell on a fairly straight line, possibly slightly curved, with activation energy ∼0.84 eV. On annealing at 400
°C. On first heating, ●, data fell on a fairly straight line, possibly slightly curved, with activation energy ∼0.84 eV. On annealing at 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C overnight, its conductivity increased (→) and variable temperature data ■, ○ fell on a second straight line with decreased Ea ∼ 0.55 eV. At T > 400
°C overnight, its conductivity increased (→) and variable temperature data ■, ○ fell on a second straight line with decreased Ea ∼ 0.55 eV. At T > 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, a further jump in conductivity occurred, –
–
–, with a new activation energy of ∼0.23 eV. In order to exclude any possible influence of the Ag electrode on these conductivity data, especially for samples heated >400
°C, a further jump in conductivity occurred, –
–
–, with a new activation energy of ∼0.23 eV. In order to exclude any possible influence of the Ag electrode on these conductivity data, especially for samples heated >400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, a second set of experiments was performed in which electrodes (of InGa alloy) were added only after the heat treatments >400
°C, a second set of experiments was performed in which electrodes (of InGa alloy) were added only after the heat treatments >400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Similar trends to those shown in Fig. 8 were obtained, thus showing that the conductivity increases were unaffected by the electrode. From the general trend of increased conductivity with increased oxygen contents, some oxidation of the sample had clearly occurred at >400
°C. Similar trends to those shown in Fig. 8 were obtained, thus showing that the conductivity increases were unaffected by the electrode. From the general trend of increased conductivity with increased oxygen contents, some oxidation of the sample had clearly occurred at >400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. However, X-ray patterns of the initial, reduced sample and after the second cooling were identical, and so oxygen uptake was probably limited to the grain boundaries, forming relatively thin conductive regions. C3 remained small and was similar to that in the as-prepared sample, Table 2. From this, we suggest that charge carriers travel along conductive surface regions, bypassing the more resistive inner bulk region, Fig. 7b; thus, C3 still refers to the bulk of the sample, which includes both the conductive surface layers and the less conductive grain cores.
°C. However, X-ray patterns of the initial, reduced sample and after the second cooling were identical, and so oxygen uptake was probably limited to the grain boundaries, forming relatively thin conductive regions. C3 remained small and was similar to that in the as-prepared sample, Table 2. From this, we suggest that charge carriers travel along conductive surface regions, bypassing the more resistive inner bulk region, Fig. 7b; thus, C3 still refers to the bulk of the sample, which includes both the conductive surface layers and the less conductive grain cores.
 | ||
| Fig. 8 Arrhenius plots and activation energies of bulk conductivities, σ3, for compositions x = 1.2: γ = 6.0, *, and γ = 5.8 during first (filled symbols) and second (open symbols) heating, ● ○, and cooling, ■ □, cycles. | ||
The activation energy decreased greatly during reoxidation of the grain surfaces, Fig. 8, so much so that, after heating >400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, it was smaller than that of the as-prepared sample. Since Ea decreases with increasing oxygen content, it seems likely that the oxygen content in the outer surface region was larger, i.e.γ > 6.0, than in the as-prepared sample. It was shown previously6 that an anomalously high oxygen content is achieved on oxidation at intermediate temperatures, ∼750 to 1000
°C, it was smaller than that of the as-prepared sample. Since Ea decreases with increasing oxygen content, it seems likely that the oxygen content in the outer surface region was larger, i.e.γ > 6.0, than in the as-prepared sample. It was shown previously6 that an anomalously high oxygen content is achieved on oxidation at intermediate temperatures, ∼750 to 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C; these are much higher temperatures than achieved during the present impedance measurements, 400 to 470
°C; these are much higher temperatures than achieved during the present impedance measurements, 400 to 470![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, which may therefore be high enough to oxidise the grain boundary/grain surfaces but not the entire sample.
°C, which may therefore be high enough to oxidise the grain boundary/grain surfaces but not the entire sample.
CaMnO3.0
A pellet of CaMnO3.0 with Mn valence state +4.0 was prepared.6 The −Z′′, Z′ plane at 83 K is given in Fig. 9 for two applied voltages, 100 mV and 1 V. The total resistance was ∼70 Ω cm and varied greatly with applied voltage; thus the arcs seen in Fig. 9 represent the electrode/sample interface. The resistance of the sample, given by the high frequency intercept of the arcs, is ∼42 Ω. The electrical properties could not be further investigated with the frequencies available and it is not known whether any grain boundary resistance contributed to the sample resistance, but certainly the bulk resistance is ≤42 Ω at 83 K. This is much lower than reported for CaMnO3 − δ by Taguchi (R ∼ 10 kΩ cm at T ∼ 160 K).8 The reason for this discrepancy is not known; it may involve differences in oxygen content in the different samples. | ||
| Fig. 9 CaMnO3.0, prepared in O2: impedance complex plane plot at 83 K, with applied ac voltages of 100 mV, ○, and 1 V, ●. | ||
Discussion
In the present family of materials, complex electrical characteristics were apparent but some general trends may be seen: (i) with increasing x, and decreasing Mn valency at γ = 6.0, both R and Ea increase, Fig. 10; (ii) with decreasing γ and Mn valency at constant x, both R and Ea increase (Figs. 6 and 10 and ref. 7). From the composition and temperature dependence of conductivity, deductions about the conduction mechanism may be made and the role of parameters that influence the conductivity may be considered.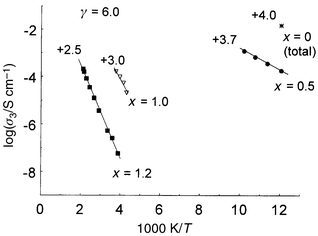 | ||
| Fig. 10 Arrhenius plots of bulk conductivities, σ3, for fully oxidised compositions, Ca2Mn2 − xNbxO6.0: x = 0.5, ●; x = 1.0, ▽; x = 1.2, ■. Total σ for CaMnO3.00, *. | ||
Conduction mechanisms
For all the materials studied, apart from CaMnO3 for which we have few data, the conductivity is thermally activated and the Arrhenius plots are generally linear. Although σ and Ea vary with composition, the conductivity pre-exponential factor A, from σ = A exp(−Ea/kT), is almost constant, Fig. 11. Hence, the number of mobile carriers is constant and the conductivity is governed entirely by the ease of hopping, Ea. The conductivity is therefore intrinsic since there is no evidence for any extrinsic region in which carrier concentration varies. Since the conductivity is thermally activated and intrinsic, the mechanism involves the hopping of localised electrons or small polarons, rather than conduction through delocalised bands. Since the carrier concentration is independent of composition, it is inappropriate to refer to the conduction mechanism as p-type, even though the conductivity increases with increasing average oxidation state of Mn. | ||
| Fig. 11 Pre-exponential factor, A, as a function of Mn valency. | ||
Mn 3d orbital interactions
The details of the conduction mechanism can be discussed in terms of electron configurations of Mn ions and possible interactions involving the 3d orbitals. If we assume a high spin configuration for octahedrally coordinated Mn, consistent with magnetic susceptibility data for Ca2MnNbOγ,7 and neglecting any Jahn–Teller splitting, then the energy level diagrams for the various Mn ions are: Mn2+: t2g3 eg2; Mn3+: t2g3 eg1; Mn4+: t2g3 eg0. It is usually assumed that the electrons responsible for conductivity are those in the highest occupied levels, since electrons in lower levels are increasingly localised or in levels that are fully occupied. For example, the electronic conduction in GMR manganates with Mn in a mixed +3/+4 valence state is explained by assuming that t2g3 electrons are localised with a spin of S = 3/2, while eg electrons are strongly hybridised with the oxygen 2p state, Fig. 12a, forming a σ d–p bond between Mn and O;1,10,11 eg electrons hop from Mn3+ to Mn4+via the bridging oxygen, so that effectively Mn3+ and Mn4+ ions change places. The difficulty in applying this model to the present materials is that conductivity is greatest when Mn is in the +4 state, for which the eg orbitals are empty. If, however, some partial occupancy of eg orbitals occurs, through contribution of oxygen 2p electrons to the σ d–p bonds, then would be forced to conclude that, effectively, oxygen 2p electrons are responsible for the high conductivity in CaMnO3. | ||
| Fig. 12 Possibilities of Mn d-orbital interactions in a simple cubic AMnO3 perovskite structure: (a) eg–pσ–eg overlap of Mn ions with oxygen (σ-bond), (b) t2g–pπ–t2g overlap of Mn ions with oxygen (π-bond) and (c) direct t2g–t2g overlap between Mn ions across the unit cell face diagonal. | ||
Alternatively, the t2g rather than the eg electrons may be responsible for the conductivity. The relative disposition of t2g orbitals on adjacent Mn atoms in a simple perovskite structure suggests two possible modes of orbital interaction.
First, t2g orbitals may overlap with the O pπ orbitals in directions parallel to the unit cell edges, Fig. 12b. This situation is comparable to that in tungsten bronzes, AxWO3 with A = alkali metals or protons,12,13 in which t2g–pπ–t2g overlap is responsible for metallic conductivity. WO3 (x = 0) has an empty W 5d (t2g) band and is insulating. Reduction of W6+ to W5+ (increasing x) introduces electrons into the 5d (t2g) band and at high x, a Mott transition to a metallic state occurs. This model may be considered for the present system in which the t2g band is half-filled for d3, d4 and d5 high spin Mn; conduction through the t2g orbitals is possible in all three cases but the materials are semiconducting rather than metallic, which might indicate that the t2g–pπ–t2g orbital overlap is poor. The increase of conductivity on oxidation could correlate with an increase in t2g–pπ–t2g orbital overlap due to reduction in unit cell dimensions. Factors that influence orbital separation are discussed later.
It might also be considered whether t2g orbitals interact directly across the unit cell face diagonal, as occurs in some rocksalt structures, e.g. TiO, Fig. 12c. In TiO, the t2g conduction band, resulting from direct t2g–t2g overlap, is 1/3 full (d2 Ti) and gives rise to metallic conduction.12 In the present system, the Mn–Mn distance across the cell face diagonal is larger than in rocksalt structures (approximately twice as long) and direct t2g–t2g overlap appears unlikely.
To a first approximation, then, it is concluded that conduction in the present materials may involve either the t2g electrons through t2g–p–t2g orbital overlap or the eg orbitals through σ d–p orbital overlap, with electrons provided primarily by oxygen. Enormous variations in conductivity are seen with changing valence of Mn; since the number of t2g and oxygen p electrons is constant, the activation energy and, hence, the conductivity must be controlled by the separation of orbitals on adjacent atoms.
Mn 3d-orbital separation
In considering the separation of orbitals on adjacent atoms, two factors are potentially important: the size/charge of Mn and the unit cell dimensions. First, the idealised cation radius of Mn in octahedral coordination decreases on oxidation, from 0.83 Å (Mn2+) to 0.53 Å (Mn4+).14 Consequently, with increasing charge of Mn, the d electrons should be held more tightly to the nucleus, the orbital extent and overlap should decrease and conductivity through the d orbitals should decrease. The exact opposite occurs, however, as the conductivity increases with Mn oxidation state. Alternatively, with increasing occupation of eg orbitals in the sequence Mn4+–Mn3+–Mn2+, the ionic size of Mn increases but the spatial extent of the t2g orbitals might decrease because of electron–electron repulsion, and hence t2g orbitals on adjacent Mn ions become more separated. The latter situation would be consistent with the present data; conductivity is lowest when all Mn is in valence state +2 and possesses the highest number of eg electrons.Second, an increase in unit cell dimensions must increase interatomic distances and, therefore, increase the orbital separation. In the present materials, the unit cell expands on increasing x and decreasing γ, both of which are associated with a decrease in Mn valence. Fig. 13 shows that activation energies increase non-linearly with the average perovskite unit cell dimension, ap = 3√V. For small interatomic distances with a higher degree of orbital overlap, Ea is relatively small and varies little with unit cell size; for large interatomic distances and poor orbital overlap, Ea depends increasingly strongly on ap. This simple correlation, Fig. 13, indicates that interatomic distance may be the main factor that determines electronic conductivity in the present system.
 | ||
| Fig. 13 Variation of Ea with perovskite unit cell dimension, 3√V. | ||
Tilting of octahedra in the GdFeO3-type structure
We may also consider the possible influence of non-linearity of Mn–O–Mn bonds due to tilting of MnO6 octahedra in the orthorhombic solid solutions with the GdFeO3 structure and the absence of tilting in the reduced, cubic solid solutions. Cubic structures with a Mn–O–Mn bond angle of 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C would maximise the Mn–O–Mn σ and π orbital interactions. In structures with tilted octahedra, the Mn–O–Mn bond angles deviate from 180
°C would maximise the Mn–O–Mn σ and π orbital interactions. In structures with tilted octahedra, the Mn–O–Mn bond angles deviate from 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, give poorer σ and π orbital overlap and increased localisation of d-electrons.15,16
°C, give poorer σ and π orbital overlap and increased localisation of d-electrons.15,16
Conductivity data do not indicate a major influence of octahedral tilting on conductivity. For x = 0.5, as oxygen is removed from the structure, the orthorhombicity decreases and a transition to a simple cubic structure occurs. At the same time, a decrease in conductivity with increased activation energy is observed which is perhaps the opposite of what would be predicted. Also, there is no obvious correlation between the degree of orthorhombicity (greatest for x = 1.2, γ = 6) and conductivity (greatest for x = 0, γ = 6).7,17,18
Oxygen vacancies
Some compositions were oxygen-deficient and contained 3–11% oxygen vacancies. The role of oxygen vacancies may be discussed in terms of eg–pσ–eg or t2g–pπ–t2g orbital overlap which, on removal of oxygen from the structure, may prevent d–p–d interactions and interrupt electron hopping between the metal ions, leading to low conductivity. This is consistent with the observed decrease of conductivity with increasing oxygen deficiency.Nb content
We can discount any major effect of inert Nb5+ ions on the conductivity, provided that there are sufficient Mn–Mn or Mn–O–Mn close contacts to give a 3D hopping pathway through the structure for electrons. Assuming a random distribution of Mn and Nb over the B-sites, the question of whether Mn–Mn or Mn–O–Mn interactions can occur depends on composition and is essentially a percolation problem; provided there is sufficient Mn present to provide a percolation pathway, then dc conduction is possible and should be fairly insensitive to the Nb content. For the present materials, the entire range, 0 ≤ x ≤ 1.2, is within the percolation limit.High spin Mn3+
We may also consider whether, in situations where all Mn is in the +3 state, any anomalies in the electrical properties are expected. There is some evidence for this in fmax data for the bulk conductivities shown in Fig. 14. The activation energy for the data set with valence state +3 is anomalously high and out of sequence with the remaining data; it could be that, for this composition, the unit cell is locally distorted, perhaps with a structural elongation associated with a local Jahn–Teller effect, and this gives rise to a small increase in Mn–Mn separations, and hence an increase in activation energy. | ||
| Fig. 14 Arrhenius plots of log fmax (obtained from M′′ or Z′′ data) for bulk responses in the Ca2Mn2 − xNbxOγ system: x = 0.5, γ = 6.0, ●, and γ = 5.34 with the two bulk responses, ○ and ●; x = 1.0, γ = 6.0, ▽; x = 1.2, γ = 6.0, ■, and γ = 5.8, □. | ||
Conclusions
The variation of conductivity with composition in the Ca2Mn2 − xNbxOγ system may be discussed in several ways. The increase of conductivity on oxidation of Mn is often explained by the concept of p-type hopping conduction,19 but this model cannot explain the high values of conductivity when Mn has valency +4.0. We found no evidence that Mn electrons in the eg orbitals are responsible for conduction. Instead, either oxygen p electrons forming σ d–p bonds with eg orbitals or t2g electrons linking through oxygen pπ orbitals are the conducting species. Direct t2g–t2g orbital interactions between Mn ions across the unit cell face diagonal are unlikely because of the large Mn–Mn interatomic distance. Conductivities depend on the degree of d–p orbital overlap, which is influenced directly by Mn–O–Mn distances and, hence, unit cell dimensions. There was no systematic dependence of conductivity on Mn–O–Mn bond angle but the octahedral tilt angles may be too small to have a significant effect.The electrical characteristics of ceramic samples depended very much on sample history. Samples that were partially reduced [in situ reduction in H2/N2 in TG experiments] were particularly complex, probably because reduction commenced at the grain surfaces, giving rise to oxygen gradients and non-equilibrium structures. Similarly, reduced samples that were allowed to partially reoxidise gave rise to samples that were electrically inhomogeneous. In both cases, impedance measurements provided a sensitive indicator of sample inhomogeneity. Most of the data reported here are for samples that were fully oxidized or fully reduced; their results provided a framework on which to explain the behaviour of more complex inhomogeneous samples of intermediate oxygen content.
References
- C. N. R. Rao, A. K. Cheetham and R. Mahesh, Chem. Mater., 1996, 8, 2421 CrossRef CAS
.
- R.-F. Huang and W. Y. Howng, J. Mater. Res., 1996, 11, 3077 CAS
.
- W. T. A. Harrison, T. H. Lee, Y. L. Yang, D. P. Scarfe, L. M. Liu and A. J. Jacobson, Mater. Res. Bull., 1995, 30, 621 CrossRef CAS
.
- B. Ma and U. Balachandran, J. Electroceram., 1998, 2, 135 CrossRef CAS
.
- P. Hagenmuller, M. Pouchard and J. C. Grenier, Solid State Ionics, 1990, 43, 7 Search PubMed
.
- A. Kruth, U. Guth and A. R. West, J. Mater. Chem., 1999, 9, 1579 RSC
.
- A. Kruth, M. Tabuchi, U. Guth and A. R. West, J. Mater. Chem., 1998, 8, 2515 RSC
.
- H. Taguchi, Phys. Status Solidi A, 1985, 88, K79 CAS
.
- J. T. S. Irvine, D. C. Sinclair and A. R. West, Adv. Mater., 1990, 2, 132 CrossRef CAS
.
- J. B. Goodenough, Prog. Solid State Chem., 1971, 5, 149 CrossRef
.
- P. D. Battle, D. E. Cox, M. A. Green, J. E. Millburn, L. E. Spring, P. G. Radaelli, M. J. Rosseinsky and J. F. Vente, Chem. Mater., 1997, 9, 1042 CrossRef CAS
.
-
J. A. Duffy, Bonding, Energy Levels & Bands in Inorganic Solids, Longman Scientific & Technical, Essex, 1990. Search PubMed
.
- N. S. P. Bhuvanesh and J. Gopalakrishnan, J. Mater. Chem., 1997, 7, 2297 RSC
.
- R. D. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751
.
- H. Taguchi, J. Solid State Chem., 1996, 124, 360 CrossRef CAS
.
- P. M. Woodward, Acta. Crystallogr., Sect. B, 1997, 53, 44 CrossRef
.
- A. Kruth and A. R. West, unpublished work..
- S. K. Mishra and S. Satpathy, Phys. Rev. B, 1998, 58, 7585 CrossRef CAS
.
- J. Töpfer, J. P. Doumerc and J. C. Grenier, J. Mater. Chem., 1996, 6, 1511 RSC
.
Footnotes |
| † Basis of a presentation given at Materials Discussion No. 3, 26–29 September 2000, University of Cambridge, UK. |
| ‡ Since C data are corrected for overall pellet geometry and the permittivity values, ε′, of different regions of the pellet are likely to be similar, then the capacitance values give an approximate idea of the thickness of each region.9 |
| This journal is © The Royal Society of Chemistry 2001 |
