Green chemistry measures for process research and development
David J. C.
Constable
a,
Alan D.
Curzons
b,
Luisa M.
Freitas dos Santos
c,
Graham R.
Geen
*c,
Robert E.
Hannah
d,
John D.
Hayler
e,
John
Kitteringham
c,
Michael A.
McGuire
d,
John E.
Richardson
e,
Paul
Smith
c,
R. Lee
Webb
d and
Marvin
Yu
d
aGlaxoSmithKline, 2200 Renaissance Boulevard, King of Prussia, PA 19406, USA
bGlaxoSmithKline, Southdownview Way, Worthing, West Sussex, UK BN14 8NQ
cGlaxoSmithKline, New Frontiers Science Park, Third Avenue, Harlow, Essex, UK CM19 5AW. E-mail: graham_geen-1@gsk.com
dGlaxoSmithKline, 709 Swedeland Road, King of Prussia, PA 19406, USA
eGlaxoSmithKline, Old Powder Mills, near Leigh, Tonbridge, Kent, UK TN11 9AN
First published on 31st January 2001
Abstract
A set of metrics has been developed which enables a simple assessment to be made of batch processes in terms of waste, energy usage, and chemistry efficiency. It is intended to raise awareness of green chemistry by providing a tool to assist chemists in monitoring progress in the reduction of environmental impact as they design new routes and modify processes.
Green ContextThe development of a set of simple measurements which help to assess the environmental impact of a chemical process is described. The impact of such an approach on both defining real areas for improvement, and highlighting the importance of green chemistry as an integral process development tool is highlighted.DJM |
Introduction
Primary manufacturing in the pharmaceutical industry involves the use of multistage batch processes to prepare relatively small to moderate quantities (by chemical industry standards) of complex chemical compounds. As a normal part of the chemical development process for drug candidates, the route of synthesis used for the preparation of initial supplies is either optimised or, more often, replaced by an alternative route, so that the final compound is prepared more efficiently.We wished to introduce a set of measures that would enable an assessment of the initial development process and allow environmental improvements to be monitored during the development stages. It was also intended that these measures would raise the awareness of green chemistry,1,2 highlight key issues and provide information that would assist chemists in choosing between alternative routes. We required a relatively simple template that could be completed using readily obtainable information such as that found in typical process description reports. This information would also need to tie in with the comprehensive environmental review already conducted on compounds in the later stages of development and align with the GlaxoSmithKline Design for the Environment programme.3
The measures are a mixture of qualitative and quantitative assessments of inputs and outputs for a particular process.4 The latter is a refinement of the E-factor proposed by Sheldon.5 Although designed specifically for use within the company, these measures should find wider relevance within the pharmaceutical and chemical industries.6
Results and discussion
The template that has been developed is shown overleaf. It is divided into four sections.Section 1: identification
This section provides information on the identity of the compound, the synthetic route (the scheme would normally be attached) and the basis of the assessment (usually a batch report).Section 2: synthetic complexity
The intention of this section is to record the number and type of operations comprising the process. It has required the development of some working definitions, which are given below.Example of the difference between a stage and a step: This may be achieved in one stage, but has three chemistry steps. 1. Reduction of nitro group 2. Reduction of aromatic ring 3. Hydrochloride salt formation
Section 3: environmental impact
This section is intended to provide information on key material usage, including solvents, quantities of waste and extreme conditions. It is used as a surrogate for energy consumption, and is a mixture of text and calculation. The assessment is made on the basis of the identity and quantity of input and auxiliary materials only and does not take into account the nature of the reaction products or the effects of side reactions.Although these numbers do not provide information on waste stream composition, they provide enough information to prompt the chemist to explore opportunities for mass reduction and recycling, and to consider the potential environmental impact of the waste.
Typically, significant reductions in volumes of solvent, aqueous and input materials are experienced as more efficient routes to target compounds are introduced and optimised. An actual example of this progress is illustrated in Fig. 1.
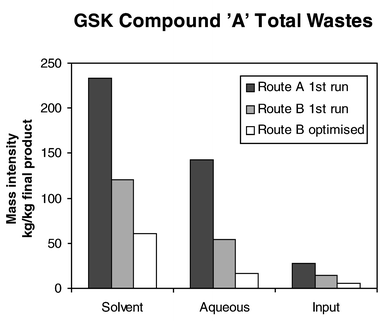 | ||
| Fig. 1 | ||
Section 4: comments
This is an opportunity to highlight the use of steps involving catalytic reagents, asymmetric processes and other types of chemistry that could be more environmentally friendly. Comments may also be added about other issues that may not have been captured but that may have significant environmental impact. For example, if the chemical nature of the product is thought to restrict opportunities for environmental optimisation or where the mass of an input material appears to be high but it may be reuseable, such as in the case of chromatography packing. This section could also incorporate a description of any assumptions that have been made when preparing the assessment.Implementation
To be of greatest value, the template must be completed at various key stages during the chemical development of the target compound. An initial assessment at the time of preparation of the first supplies (usually on a large laboratory scale) will provide a baseline. Thereafter, updates are required when preparing the first pilot plant batch using the initial chemistry, when a new route is introduced into the plant, or if there are significant process improvements made without a change of route.We have found that it normally takes no more than 1–2 hours to complete the first template for a compound. Subsequent updates may be completed more quickly. It is important to emphasise that while these measures give a broad view of the likely environmental impact of the process, the information derived is limited. In particular, the system does not provide a breakdown of the composition of waste streams, nor does it address treatment options, or the acquisition of raw materials from sustainable sources. Key inputs to the design of the process from the process engineering and safety testing groups are also outside the scope of the current system. When processes are transferred to a manufacturing plant, it is also essential that a full environmental assessment is undertaken and this must be completed prior to the preparation of validation batches.
The introduction of the set of measures described here has raised the environmental awareness of our process chemists and has enabled us to document and highlight improvement opportunities. These opportunities will help move GlaxoSmithKline towards achieving the ultimate target of drug manufacture using ‘green chemistry’. It will also enable a direct comparison of the potential environmental impact of alternative routes for a particular compound as well as between different compounds.
References
- Green Chemistry: Theory and Practice, ed. P. T. Anastas and J. C. Warner, Oxford University Press, Oxford, 1998. Search PubMed.
- J. H. Clark, Green. Chem., 1999, 1, 1 RSC; D. J. Dale, P. J. Dunn, C. Golightly, M. L. Hughes, P. C. Levett, A. K. Pearce, P. M. Searle, G. Ward and A. S. Wood, Org. Process Res. Dev., 2000, 4, 17 Search PubMed.
- A. D. Curzons, D. J. C. Constable, D.N. Mortimer and V. L. Cunningham, Green Chem., 2001, 3, 1 Search PubMed.
- C. E. Berkoff, K. Kamholz, D. E. Rivard, G. Wellman and H. Winicov, Chemtech, 1986, 16, 552 Search PubMed.
- R. A. Sheldon, Chem. Ind. (London),, 1992, 903 Search PubMed; R. A. Sheldon, Chem. Ind. (London), 1997, 12 Search PubMed.
- Details of The Centre for Waste Reduction Technologies sustainability metrics may be found at the website, http://www.aiche.org..
- A. D. Curzons, D. C. Constable and V. L. Cunningham, Clean Products Processes, 1999, 1, 82 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |


