So you think your process is green, how do you know?—Using principles of sustainability to determine what is green–a corporate perspective
Alan D.
Curzons
*a,
David J. C.
Constable
b,
David N.
Mortimer
a and
Virginia L.
Cunningham
b
aGlaxoSmithKline, Southdownview Way, Worthing, West Sussex, UK BN14 8NQ. E-mail: alan.d.curzons@gsk.com
bGlaxoSmithKline, 2200 Renaissance Boulevard, King of Prussia, PA 19406, USA
First published on 31st January 2001
Abstract
An approach to quantitatively and systematically evaluate synthetic organic reactions and processes is described. This sustainability-based approach allows chemists to clearly assess whether or not chemistries and chemical processes are ‘greener’. The results of this work indicate that close attention to effective use and reuse of solvents will result in the largest gains for reducing life cycle impacts in batch chemical operations.
Green ContextWe all work on green chemistry, but are we really sure about that? This is the first of two articles in this issue to discuss the development of a set of criteria designed to answer just this question. The authors describe their approach in this article by discussing the framework in which chemistry is carried out, and the necessary factors which need to be considered from an industrial perspective. The paper introduces the company’s green metrics, by which an assessment of ‘greenness’ can be made.DJH |
Sustainable Chemistry Defined (OECD Workshop on Sustainable Chemistry, 1998) 4‘Within the broad framework of Sustainable Development, we should strive to maximise resource efficiency through activities such as energy and non-renewable resource conservation, risk minimisation, pollution prevention, minimisation of waste at all stages of a product life-cycle, and the development of products that are durable and can be re-used and recycled. Sustainable Chemistry strives to accomplish these ends through the design, manufacture and use of efficient and effective, more environmentally benign chemical products and processes.’ |
The sustainable development context
Many people over the past decade have been discussing how to change the way humans live and behave to reduce their impacts on the environment, promote economic development, and enhance social welfare. Under the broad umbrella of Sustainable Development, different approaches to the usual way of living and behaving are being discussed, and in some instances, implemented. Sustainable Development, originally defined by the Brundtland Report in 1987,1 is being seriously considered and debated in national and local government policy arenas, corporate boardrooms and academia. The idea that the current generation should live in a way that does not impact future generations’ ability to live as well, if not better, than we do—for all living beings—is not necessarily a new idea, but one that has generally been ignored by modern industrial societies.So why and how does a Company, who many would say has a primary objective only to add shareholder value, implement the principles of sustainable development in an area as specialized as synthetic organic chemistry, and how does this relate to the concept of green chemistry? The first question is why. Adding shareholder value and rigorously pursuing the principles of sustainable development are not mutually exclusive. Although it is beyond the scope of this article and offered without debate here, the appearance of several recent investment funds (Innovest and the SAM Sustainability Group) suggest that increased shareholder value is in fact associated with companies that pursue Sustainable Development.
Apart from adding shareholder value, why else might a company want to consider using principles of Sustainable Development as it considers what is green chemistry? There are several reasons.
First, there are risks to the business from unsustainable business practices. These include risks from greenhouse gas emissions taxes (energy, transportation), pollutants and toxic releases (energy, VOCs, various chemical compounds), shipment of highly hazardous materials (reagents, intermediates, raw materials, solvents), new and increasingly restrictive regulations (air, water, land, hazardous waste), etc. Second, there is the desire for competitive advantage. Companies that reduce cost by decreasing mass intensity (the total amount of mass required to produce a unit of product or service, usually on a wt/wt basis) or energy intensity (the total amount of energy required to produce a unit of product or service) will be more profitable. The more adept a company is at implementing technologies and new chemistries that simultaneously reduce mass and energy intensity, the more it will realise higher profits. They will also generally have fewer risks from unsustainable business practices. Finally, every company should be concerned about the local and business community in which it operates, and maintaining its ‘right-to-operate’ in these communities.
The second question of how a company implements Sustainable Development principles in the synthetic organic and green chemistry context is a bit more difficult. Over the past several years we have been considering what a company such as GlaxoSmithKline might do if it is to move towards more sustainable business practices. A logical place to start is with a consideration of our impacts to the environment. The companion dimensions of Sustainable Development, economic and social development, are admittedly longer term and more difficult issues to develop and implement at this time. This does not mean that they have no place in the green chemistry context, it is just more difficult to arrive at a consensus for the best way forward. An example of the complexity of the economic and social dimensions may be illustrated in the case of harvesting a natural product used as a synthetic precursor for an anti-cancer drug. The compound might be harvested from a plant or marine organism found in a developing country. Sorting out the various issues to ensure appropriate economic remuneration (just and sufficient for the benefit derived by the business), protection of local resources (human and environmental), impacts to the community from changes to the economy, etc., can be particularly daunting, and is certainly beyond the scope of this journal.
It has been our experience that most companies are very interested in the concept of Green Chemistry, but few have a good sense of how to move it forward. From a pragmatic standpoint, we are pursuing a largely environmental approach as a first step towards Sustainable Business Practices. This article attempts to outline in broad terms the approach that we are taking at the Corporate level within GlaxoSmithKline. It is offered in the hope that it may promote discussion and begin to build a consensus on how to know a process is Green. A companion article2 describes how the Chemical Development Department at GlaxoSmithKline Research and Development is implementing a set of metrics to raise awareness of Green Chemistry. The R&D metrics are considered to be a ‘grass-roots’ complementary effort to the overall Corporate effort.
The design for the environment tool kit
Given that we are starting with the environment, what might the typical bench and management level scientist or engineer require to assist them in making the best possible decisions—those that are the most ‘Green?’ Current thinking suggests that the best way to make a product ‘Green’ is to consider what needs to be done during the design phase to ensure that the product being produced has as few adverse impacts as possible while maximizing the benefit it brings to society. This is the so-called ‘Design for the Environment’ approach. In the green chemistry context, we might understand this to mean producing complex molecules in just a few room-temperature atom economical (only A + B making only C) synthetic steps, using non-hazardous reagents and intermediates (derived from renewable feed stocks), in the absence of solvents or in solvents that are 100% recoverable, with no adverse safety issues. All of this is to be accomplished using the least amount of energy possible that also happens to be produced from renewable energy sources.To meet this need, we have begun to assemble a ‘Design for the Environment’ Tool Kit. The first step in assembling the Tool Kit was to develop a set of fundamental and broadly applicable metrics.
Since 1997, 10–15 chemical and allied industry companies that are a part of the American Institute of Chemical Engineers’ Center for Waste Reduction Technologies (AIChE/CWRT) have been working to develop a set of core and complementary sustainability metrics. The CWRT group built on the foundation of work completed by the National Roundtable on the Economy and Environment (NRTEE), a Canadian funded effort undertaken by about a dozen manufacturing companies (chemical, telecommunications, mining, utility, etc.). The CWRT also generally adopted the eco-efficiency framework to Sustainable Development that was developed by the World Business Council for Sustainable Development as a first step to guide the development of their metrics. A description of the CWRT efforts and the metrics that have been piloted may be found at http://www.aiche.org/CWRT. We (GlaxoSmithKline) have refined these metrics and are using them to organize our thinking about several programmes under development.
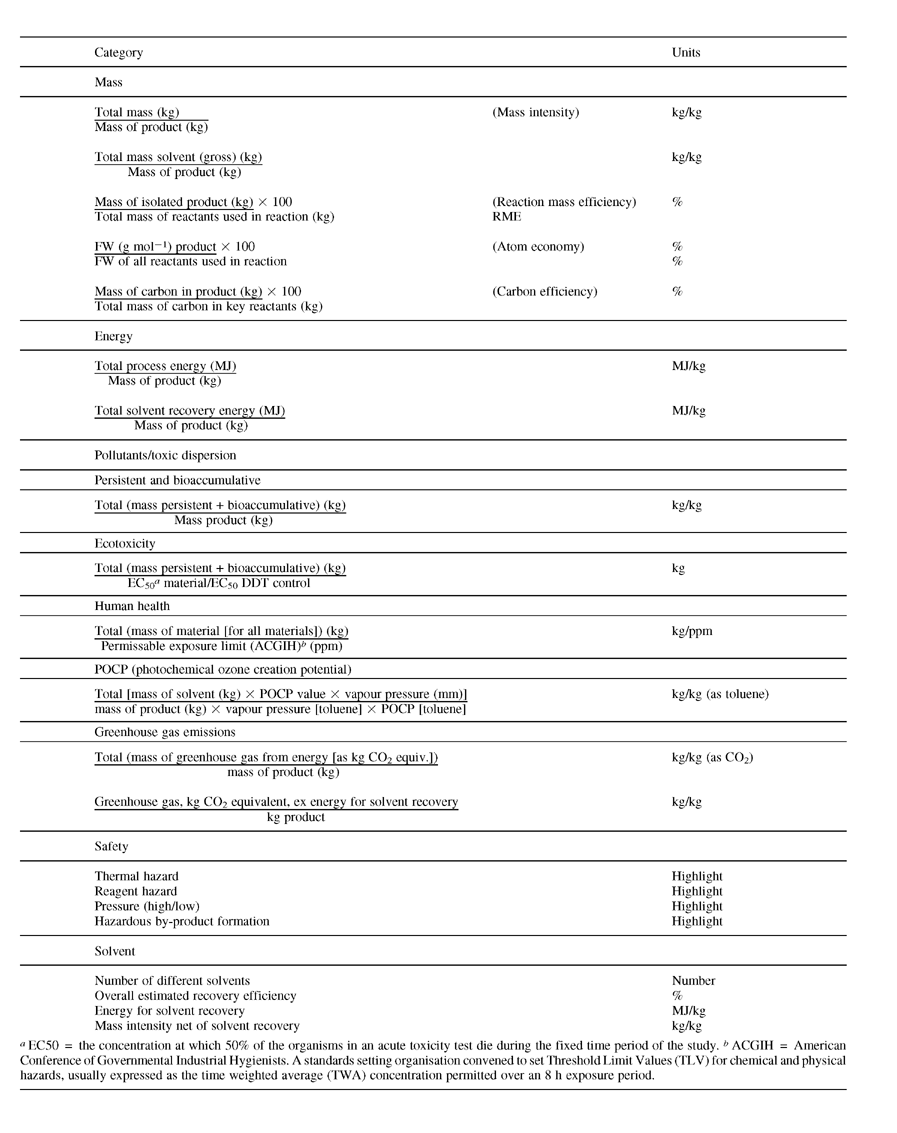
Beside metrics, what else do we need in the toolkit? Firstly we have embarked on a significant programme in Life Cycle Inventory/Assessment (LCI/A). The ultimate intention is to develop guidance which will enable scientists to assess the lifecycle burdens, from cradle to gate, associated with their processes and to be able to compare these during route selection. As a first step to achieve this, we have been working to create an impact inventory for one of our major drug substances. This inventory relates to about 115 chemicals and is being organized into impact categories based on our sustainability metrics; i.e., mass intensity, energy, pollutants and toxics.
Secondly, especially given our business context, we have a programme for developing a Total Cost Assessment (TCA) methodology as a means of assigning a monetary value to our Life Cycle impacts and making better pollution prevention decisions. This is also a collaborative programme with the CWRT and a detailed description of the methodology and data may be obtained from the above website. Ultimately the TCA data will be used in conjunction withthe LCI database of chemicals to develop a LCI/A tool for our bench level scientists and engineers.
We have also embarked on a detailed Green Chemistry programme that is described below. The final tool we have just begun to develop is best described as Green Technology guidance. This Green Technology guidance will help scientists and engineers to choose the best technology (unit operations) from a sustainability metrics perspective.
Taken together, the ‘Design for Environment’ Tool Kit will provide a very powerful tool for moving GlaxoSmithKline towards more sustainable business practices. It is critically important to note that we consider all the component parts of the Tool Kit in our decision-making practices. We have found that each of these Tools informs the other tools and influences the decisions that are made. The absence of this broad approach could very well lead to perverse answers during decision making. It should also be noted that this is a long-term strategic initiative that will have an eventual but impressive business benefit.
The green chemistry programme
So how do we ensure that a series of complex synthetic organic reactions, that ultimately become a synthetic process, are ‘green’ and how do we measure success in achieving this? Paul Anastas and John Warner3 produced a seminal set of twelve principles that initiated the green chemistry concept and have guided many in their pursuit of what is ‘green’. In addition, the OECD Workshop on Sustainable Chemistry provided an excellent summary of sustainable chemistry (see box). The twelve principles and the OECD Sustainable Chemistry definition are very helpful in challenging chemists to consider what they might do to make their processes greener.While the principles and the OECD definition are very good at setting the context, we desired to establish a systematic methodology and framework that would provide a means to evaluate, assess and organize our thinking. Given our work with sustainability metrics, we pursued a metrics-based approach to determining what is ‘green.’
The first step, and a difficult one at that, was to collect descriptions of our chemistries that have been used over the past ten years. This task was aided by the regulatory requirement to produce a description of how active pharmaceutical ingredients are produced. We collected about two hundred individual chemical reactions for about 38 different products, some of which are now commercial products. Each of these reactions was categorised in several different ways in an attempt to make it easier for chemists to understand and search for the chemistry that was being evaluated, and its purpose. Table 1 contains an illustration of the approach for several categorisations. The first column defines the purpose of the synthesis, the second provides the reaction category, and the third provides the reaction name or type. While this particular approach to categorisation made sense to GlaxoSmithKline, there may be many ways to categorise reactions.
| Purpose | Reaction category | Reaction name/type |
|---|---|---|
| Forming a new carbon–oxygen bond | O-Alkylation | Ether synthesis |
| Forming a new carbon–carbon bond | C-Alkylation | Alkylation of an aromatic |
| Forming a new carbon–carbon bond | Addition to C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O O |
Knoevenagel |
| Forming a new carbon–nitrogen bond | N-Acylation | Amidation |
| Forming a new carbon–nitrogen bond | N-Alkylation | Of heterocycle |
| Forming a new carbon–nitrogen bond | N-Alkylation | Of amine |
| Forming a new carbon–sulfur bond | S-Alkylation | Thioether synthesis |
| Reduction | Catalytic hydrogenation/hydrogenolysis | |
| Reduction | Metal hydride | Lithal |
| Cyclisation | Heterocycle synthesis | By miscellaneous ring closure |
| Elimination | C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) C formation C formation |
|
| Hydrolysis | Acid catalysed | |
| Hydrolysis | Base catalysed | |
| Halogenation | Halogenation of an alcohol | |
| Salt formation | Acid or base | |
| Neutralisation | ||
| Resolution | Using diastereoisomers | Acid or amine |
The development and use of the metrics for assessing individual reactions had two component parts. First, we have explored core and complementary metrics from a ‘green’ perspective that include safety and operational parameters. This approach helped to further our understanding of the chemistries and the most important ‘green’ factors that make them different. It is important to point out that this is an ongoing and iterative process. Second, we have developed a comprehensive set of heuristics that permits us to derive data for each metric from the available information in the process descriptions. Table 2 contains illustrative (not our final set) metrics of the type we have considered.
The next step was to assess the chemistry using our metrics and heuristics, and we developed an expert system, based in Microsoft Excel, to facilitate this complex and time-consuming process. Using this we have been able to evaluate all two hundred chemical reactions using the above metrics.
So now that we have our metrics set and we have completed our evaluation, what about the data and how do we use it? We have a vast amount of information that is providing tremendous insight across a range of areas. The following is intended to provide a snapshot of the output, with some selected examples covering some of the key learning points.
We have chosen to organise our thoughts according to a long, medium and short-term paradigm. Stated slightly more euphemistically, we can think of this as the pursuit of atom economy, the marriage of chemistry and engineering, and getting your house in order.
The idea of atom economical reactions, first introduced by Professor Barry Trost.5 may be a useful concept in helping to promote thinking around green chemistry. Analysis of our data demonstrates that the way in which chemists build, couple molecules, introduce chirality, etc. often employ atom inefficient reactions. Stated differently, you can’t keep throwing away large amounts of reactants and reagents in the process of making a new compound. This is both costly and has an immense Life Cycle burden associated with it. Synthetic chemists need to pursue a new set of reactions. There are two component parts to this: (1) the need to pick the most atom efficient reactions from the currently available reactions and (2) the need to develop new chemistries or new more atom economical ways of carrying out current reactions. The latter is clearly a longer term but ongoing requirement and will require close co-operation between academia and industry.
Although the idea of atom economy may be important in focusing long-term effort towards the development of new chemistry and processes, we offer one important consideration. When considering what is ‘green’, atom economy is not the only metric to consider. A plot of atom economy vs. mass intensity is shown in Fig. 1 using average data for each of the chemistries.
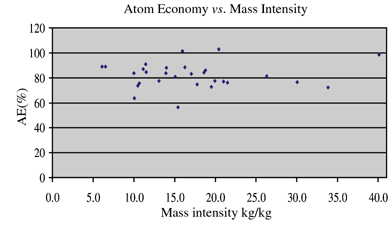 | ||
| Fig. 1 Relationship of atom economy to mass intensity. | ||
As can be seen from this plot, our data suggest that there is no correlation between atom economy and the critical mass intensity metric. The data therefore suggests that atom economy alone will not deliver the desired ‘green’ or ‘cleaner’ processes we might use to assemble molecules.
For this reason we have developed and explored several related measures known as carbon efficiency and what we refer to as reaction mass efficiency (RME). RME takes into account yields, the actual molar quantities of reactants, and the concepts of atom efficiency.
Fig. 2 provides a calculation of RME, atom economy and mass intensity while Table 3 compares atom economy and RME for 28 different chemistries. As can be seen, RME may be a more realistic metric to illustrate how far from ‘green’ we are currently operating our processes. The information presented in Table 3 is averaged data; however, in practice there will be a range of values. While a more detailed analysis of this range of values would be essential to fully understand the relative efficiencies of individual chemistries, this is outside the scope of this article.
 | ||
| Fig. 2 | ||
| Chemistry type | Atom economy (%) | Reaction mass efficiency (%) | Chemistry type | Atom economy (%) | Reaction mass efficiency (%) |
|---|---|---|---|---|---|
| Resolution | 40 | 31 | Epoxidation | 83 | 58 |
| N-Dealkylation | 64 | 27 | Bromination | 84 | 63 |
| Elimination | 72 | 45 | Hydrogenation | 84 | 74 |
| N-Alkylation | 73 | 60 | S-Alkylation | 84 | 61 |
| Chlorination | 74 | 46 | O-Arylation | 85 | 58 |
| Borohydride | 75 | 58 | N-Acylation | 86 | 62 |
| Lithal | 76 | 52 | Amination | 87 | 54 |
| Grignard | 76 | 42 | C-Alkylation | 88 | 61 |
| Hydrolysis (acid) | 76 | 50 | Iodination | 89 | 56 |
| Cyclisation | 77 | 56 | Knoevenagel | 89 | 66 |
| Cyanation | 77 | 65 | Sulfonation | 89 | 69 |
| Decarboxylation | 77 | 68 | Esterification | 91 | 67 |
| C-Acylation | 81 | 51 | Base salt | 100 | 80 |
| Hydrolysis (base) | 81 | 52 | Acid Salt | 100 | 83 |
It is important to also consider energy use, although this is also outside the scope of this article.
This leads into the middle term objectives, or alternatively, the marriage of chemistry and engineering. Stated colloquially, if you’re a chemist, take an engineer to lunch; if you’re an engineer, take a chemist to lunch. We will not be able to move effectively towards green chemistry unless there is a very close collaboration between the chemist and engineer. We say this because our data and other experience suggests that where a reaction takes place (i.e., the size, configuration, and composition of the reactor) and how the resulting intermediate or product is isolated are very important factors controlling the ‘greenness’ of a process.
For example, most chemists tend to focus on reactions rather than the technology around the reaction and virtually all reactions are undertaken in batch reactors. Thus, in general, if a reaction does not ‘work’, chemists are more inclined to change the reaction rather than investigate different equipment in which to perform the reaction. Issues of mass and energy (heat/cool) transfer, mixing, phase transfer, and general reactor design, etc., are generally not as rigorously pursued by the synthetic organic chemist as by the engineer. If these issues are not adequately considered, they may result in rather large inefficiencies during development. Given increased and ever increasing pressures to reduce time to market, and the diversity of products introduced to market, chemists simply must work with engineers in a collaborative manner to solve the problems associated with mass and energy inefficiency. This will require an understanding of the alternative technologies that are available, their relative benefits and ‘greenness’, and the widespread availability of appropriate bench scale equipment.
The final green chemistry considerations are the short-term initiatives, or “getting your house in order.” The major focus of the short term is to pay attention to solvent use. Our data demonstrates that the largest component of the mass intensity is due to reaction and work-up solvents. Solvents have a considerable life cycle impact in addition to their impacts through use and final disposal. While the market cost of solvents is quite cheap today, the broader total costs (resource depletion, life cycle and societal) are not. One illustration of the type of information we are gleaning from our evaluations is as follows:
In discussions with synthetic organic chemists, we are often told that solvent choice is dictated by the chemistry in use, and we ought not to restrict solvent choice. With a data set containing approximately 373 uses of solvents in reactions used to synthesize 38 different complex drug substances, it may be possible to draw several conclusions regarding solvent choice. Fig. 3 is a pie chart showing the mixture of solvent classes and their percentage usage. As can be seen from the chart, 50% of the solvent usage is found in two classes of solvents: alcohols and aromatics. With the addition of polar aprotics, ethers, and esters, 78% of all solvent usage is accounted for.
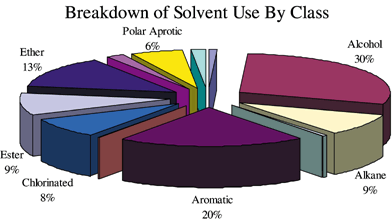 | ||
| Fig. 3 Breakdown of solvent use by class | ||
On the basis of the overall data, it might be argued that a choice of five ‘best-of-class’ solvents, one solvent in each category, may be a good starting point for running reactions when a solvent in one of these classes is called for. A simple example of this might be the case where the literature reaction calls for methanol and xylene, and the ‘best-of-class’ solvents are determined to be isopropyl alcohol (IPA) and toluene. The reactions then, could be run first in IPA and toluene. The elimination of multiple solvents has a profound effect in managing those solvents throughout their life cycle, and the total cost of managing 8 to 10 solvents is considerably more than managing five.
One of the main outputs of our data analysis is the ability to distinguish, compare and contrast different chemistries and this will eventually form part of the guidance we intend to develop for all our scientists. While it is obvious that a Knoevenagel reaction is different and is employed for different purposes than a base hydrolysis or hydrogenation reaction, it is still of great benefit to evaluate those factors that make a particular type of chemistry ‘greener’ than another type. The data also provide a baseline set against which one is able to evaluate alternative chemistries to accomplish a hydrogenation, or formation of a C–C bond, or the isolation of a free acid or base.
Summary and conclusions
We believe we have developed a reliable methodology for evaluating synthetic organic reactions through the lens of sustainable practices. Our studies generally indicate that mass and energy appear to be good leading indicators of overall environmental impact, although toxicity metrics are still in great need. In the short term, rigorous management of solvent use is likely to result in the greatest improvements to making processes ‘greener.’ Finally, the evaluation of chemistries using our sustainability metrics framework clearly differentiates between chemistries and chemical processes.References
- World Commission on Environment and Development, Our Common Future, Oxford University Press, Oxford, 1987. Search PubMed.
- D. J. C. Constable, A. D. Curzons, L. M. Freitas dos Santos, G. R. Green, R. E. Hannah, J. D. Hayler, J. Kitteringhan, M. A. McGuire, J. E. Richardson, P. Smith, R. L. Webb and M. Yu, Green Chem., 2001, 3, 7 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998. Search PubMed.
- OECD Workshop on Sustainable Chemistry, 1998..
- B. M. Trost, Science, 1991, 254, 1471 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |
