Speciation of arsenic using solid phase extraction cartridges†
Serife Yalçin and X. Chris Le*
Environmental Health Sciences Program, Department of Public Health Sciences, Faculty of
Medicine, University of Alberta, Edmonton, AB, Canada T6G
2G3. E-mail: XC.Le@UAlberta.CA
First published on 4th January 2001
Abstract
Various solid phase extraction (SPE) cartridges were investigated for speciation of arsenite [As(III)], arsenate [As(V)], monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA). Cartridges containing different types of sorbent materials were tested for arsenic retention and elution characteristics. Alumina cartridges were found to completely retain all the four target arsenic species, and are suitable for removal and preconcentration purposes. For speciation analysis, different arsenic species were separated on the basis of their selective retention on and elution from specific cartridges. DMA was retained on a resin-based strong cation exchange cartridge and eluted with 1.0 M HCl. MMA and As(V) were both retained on a silica-based strong anion exchange cartridge and sequentially eluted with 60 mM acetic acid (for MMA) and 1.0 M HCl [for As(V)]. As(III) was not retained on either cartridge and remained in solution. Arsenic species in solution and those eluted from the cartridges were subsequently quantified by using flow injection with hydride generation atomic fluorescence spectrometry (FI-HGAFS) and hydride generation atomic absorption spectrometry (FI-HGAAS). A detection limit of 0.05 µg L−1 arsenic in water sample was achieved using HGAFS. An application of the method was demonstrated at a drinking water treatment facility. As(III) and As(V) species were determined in water at various stages of treatment. The method is suitable for routine determination of trace levels of arsenic in drinking water to comply with more stringent environmental regulations.
Introduction
Arsenite, arsenate, monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) are common arsenic compounds present in natural waters.1–4 Exposure of the general population to arsenic occurs mainly through arsenic present in drinking water and food.1,5,6 Chronic ingestion of high levels (several hundred µg L−1) of arsenic in drinking water has been associated with an increased risk of developing skin, bladder, and lung cancers as well as having other non-cancerous effects.1,7–13 The US EPA has recently proposed a reduction of the maximum contaminant level (MCL) for arsenic in drinking water from its current level of 50 µg L−1 down to 5 µg L−1.14 Health Canada has also put the Canadian guideline (25 µg L−1)15 up for review. Technologies for routinely monitoring arsenic in drinking water and for removing arsenic from drinking water supplies are required to comply with the more stringent regulations.A variety of techniques have been reported for the speciation of arsenic. HPLC separation coupled with sensitive detection, such as inductively coupled plasma mass spectrometry (ICP-MS),16–22 atomic spectrometry with a hydride generation interface,23–28 and electrospray/nanospray mass spectrometry,29–34 have been shown to be most useful for arsenic speciation research. However, a study involving 25 commercial and water utility laboratories has determined that the practical quantifiable level (PQL) for arsenic in drinking water is 4 µg L−1.35 This is insufficient to comply with the more stringent environmental regulations for arsenic.
The objective of this research was to develop a sensitive and inexpensive method that can be used for routine analysis of As(III) and As(V) species in water. Our approach was to investigate the use of solid-phase extraction cartridges for speciation purposes. These disposable cartridges are inexpensive and can potentially provide specificity for selective retention of arsenic species. We report on selective retention of various arsenic species on specific cartridges followed by selective elution of arsenic separation. HGAFS provides highly sensitive detection of the specific arsenic species.
As(V) is relatively easy to remove from water by using processes such as ion exchange.36 As(III) exists as a neutral species at pH < 9 and needs to be oxidized for removal by ion exchange. Thus, it is important to determine arsenic species in water to optimize treatment processes. The method described here is suitable for routine monitoring of arsenic species in drinking water.
Experimental
Instruments
An atomic absorption spectrometer (Model SpectrAA-5, Varian, Victoria, Australia) and an atomic fluorescence detector (Excalibur 10.003, PS Analytical, Kent, UK) were used with hydride generation and flow injection analysis. In the atomic absorption system, an electrically heated quartz absorption tube with a temperature controller module, ETC-60 (Varian), was used for the atomization of the arsenic hydrides.37 An arsenic ultra lamp (193.7 nm) was operated at 10 mA using an external control module (Varian). In the atomic fluorescence detection system, an argon/hydrogen flame was used for the atomization of the arsenic hydrides.38 The hydride generation conditions were optimized for maximum sensitivity of the four arsenic species. An HPLC-HGAFS system used for the determination of arsenic species in urine has been described previously.26,39Resin-based strong cation exchange cartridges were obtained from Alltech (Ontario, Canada) and silica-based anion exchange cartridges were obtained from Supelco (Bellefonte, PA, USA). A method development kit, containing C18, (reversed-phase), silica (normal phase), florosil (weakly basic, normal phase), CN and diol (silica-based, polar phase), QMA (strong anion exchanger), NH2 (weak anion exchanger), alumina-A, -B, and -N (normal phase in acidic, basic, and neutral activity) cartridges, was obtained from Millipore–Waters (Missisauga, ON, Canada). Cartridges were preconditioned with 50% methanol and deionized water before use. A four channel peristaltic pump (Minipuls 3, Gilson, Middleton, WI, USA) with adjustable speed was used to push sample flow through the cartridges.
Standards and reagents
Sodium arsenite (Aldrich, Milwaukee, WI, USA), sodium arsenate (Aldrich), sodium monomethylarsonate (Chem Service, PA, USA) and dimethylarsinic acid (Aldrich) were prepared in deionized water.39,40 Sodium borohydride (Aldrich) solutions were prepared fresh daily, and were supplemented with 0.1 M sodium hydroxide. Elution buffers were prepared in deionized water obtained from a Maxima ultra-pure water system (USF Elga, Buckinghamshire, UK).HPLC analyses were performed by using an ODS(3), 250 × 4.6 mm, 5 µm particle size column (Phenomenex, Torrance, CA, USA). The mobile phase consisted of 5 mM tetrabutylammonium hydroxide, 1 mM malonic acid and 5% methanol at pH 5.5. All HPLC eluents were prepared in deionized water and filtered through a 0.45 µm membrane. All the reagents were of analytical grade.
Retention of arsenic species on cartridges
15–20 mL of standard solution or water sample was allowed to flow through serially connected strong cation exchange (SCX) and strong anion exchange (SAX) cartridges. A peristaltic pump was used to deliver the solution at a flow rate of 1–2 mL min−1. Only DMA was retained on the SCX cartridge, while As(V) and MMA were retained on the subsequent SAX cartridge. The cartridge effluent, which contained As(III), was collected for subsequent analysis. When the As(III) concentration was below the detection limit, an alumina cartridge was used for preconcentration of As(III).Elution of arsenic species from cartridges
After a sample passed through the cartridges, they were separated and each was eluted with 2–3 mL of appropriate eluent. Another 2–3 mL of the eluent was allowed to flow through the cartridges for a second time to verify a complete elution. The eluents were subsequently analyzed for their arsenic content by using FI-HGAAS or FI-HGAFS. In order to eliminate variations in the signal intensities due to different acid media, standard solutions were prepared in the corresponding eluents.Results and discussion
Retention and elution behavior of arsenic species on solid phase extraction cartridges
A variety of cartridges containing different packing materials were screened for quantitative retention of the four target arsenic species. Initially, a method development kit (Millipore-Waters), containing C18, silica, florosil, QMA, NH2, CN, diol, and alumina-A, -B, and -N cartridges, was used. For the determination of retention of arsenic on the cartridges, standard solutions containing 10 µg L−1 each of As(III), As(V), MMA and DMA were loaded onto each cartridge at a 1–2 mL min−1 flow rate. The amount of arsenic detected in the cartridge effluent compared with that in the standard solution represents the unretained portion of mixed arsenic species. The difference in arsenic concentrations in the solutions before and after passing through the cartridge provides information on the amount of arsenic retained on the cartridge. No retention of arsenic species was observed from the C18, silica, florosil, NH2, CN and diol cartridges. QMA retained 80% of the arsenic in the mixture. All three types of alumina cartridge (A, B, and N) showed complete retention of the four arsenic species.Elution of arsenic species from alumina cartridges
We attempted to elute each arsenic species selectively from the alumina cartridges by using several elution buffers. The results are summarized in Table 1 and are the mean ± standard deviation (s) from triplicate analyses. It was very difficult to elute inorganic arsenic from the alumina cartridges although MMA and DMA were eluted almost completely (88% and 100%) with the use of NaOH and NaCl mixture. For the elution of As(V) and As(III) 2.0 M hydrofluoric acid was necessary. Because of the differences in elution behavior, MMA and DMA can be separated from inorganic As(III) and As(V) using an alumina cartridge and selective elution.| Elution buffer | As(III) | As(V) | MMA | DMA |
|---|---|---|---|---|
| a Not examined. | ||||
| 0.1 M Phosphate, pH 7.0 | 0 | 0 | 14 ± 1 | 15 ± 1 |
| 0.1 M Succinic acid, pH 5.6 | 0 | 0 | 18 ± 1 | 30 ± 2 |
| 0.1 M Acetic acid, pH 2.5 | 0 | 0 | 5 ± 0.5 | 3 ± 0.5 |
| 1 M Hydrochloric acid | 0 | 0 | 4 ± 0.5 | —a |
| 3 M Hydrochloric acid | 0 | 10 ± 0.5 | —a | —a |
| 2 M Sulfuric acid | 0 | 18 ± 1 | —a | —a |
| 0.1 M NaOH and 0.5 M NaCl | 0 | 0 | 88 ± 4 | 100 ± 5 |
| 2 M Hydrofluoric acid | 90 ± 5 | 90 ± 5 | 100 ± 5 | 100 ± 5 |
Preconcentration of arsenicals on the alumina cartridges
The qualitative retention of arsenicals on the alumina cartridges forms the basis for preconcentration of the four target arsenic species. A urine sample (20 mL) was passed through an alumina-N cartridge. Determination of arsenic in the cartridge effluent indicated that approximately 85% of the arsenic was retained on the cartridge. The incomplete retention of arsenic is probably due to the high salt content of the urine matrix. Hydrofluoric acid (2.0 M) was used to elute arsenicals from the cartridge. The HF effluent was evaporated to dryness and diluted to 1.0 mL with deionized water. Subsequently, this sample was analyzed by HPLC-HGAFS. Speciation of arsenic in the urine sample before [Fig. 1(a)] and after [Fig. 1(b)] the preconcentration procedure is shown in the chromatograms obtained from HPLC-HGAFS analyses. As a result of a 20-fold concentration of arsenic species, previously undetectable arsenicals in the original urine sample, e.g., As(III) and MMA, became detectable.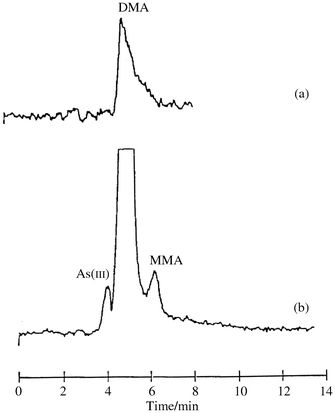 | ||
| Fig. 1 HPLC-HGAFS analyses of a urine sample before (a) and after (b) preconcentration using an alumina-N cartridge. The HPLC column was ODS(3) (250 × 4.6 mm, 5 µm particle size). The mobile phase contained 5 mM tetrabutylammonium hydroxide, 1 mM malonic acid and 5% methanol, pH 5.5. | ||
Although this approach could be useful for enrichment of low levels of arsenic species, the use of corrosive HF as an eluent affects the subsequent HPLC analysis, because HF can damage HPLC columns. The HF needs to be evaporated and the sample redissolved in deionized water prior to HPLC analysis.
Selective retention and elution of arsenic species on ion exchange cartridges
The ion exchange cartridges that showed incomplete retention of the arsenic mixture were further investigated for retention of individual arsenic species. Both silica and polymeric resin types of cation and anion exchange cartridges were tested. Some of the cartridges were able to completely retain one or two specific arsenic species, while others showed partial retention of each arsenic species. The retention for each arsenic species on these ion exchange cartridges is summarized in Table 2. Results are mean ± s (n = 6) from triplicate analyses of each eluent from duplicate retention experiments.| Cartridge type | As(III) | As(V) | MMA | DMA |
|---|---|---|---|---|
| a From Millipore–Waters. Alumina-A,-B,-N: hydrophilic, acidic, basic or neutral phase, 1.7 g sorbent. Sep-Pak Plus QMA: hydrophilic, basic anion exchange phase, 360 mg sorbent.b From Supelco. Silica-based, syringe-type cartridges. WCX (weak cation exchange): carboxylic acid, Na+ counter-ion; SAX: quaternary amine, Cl- counter-ion; SCX: aliphatic sulfonic acid, Na+ counter-ion. Sorbent materials (500 mg) were 40 µm in diameter with 60 Å pore size.c From Alltech. SCX: styrene divinyl benzene sulfonic acid, counter-ion H+, 35–500 mg sorbent, 75 µm particle size. | ||||
| Alumina-A, -B, and -Na | 100 ± 2 | 100 ± 2 | 100 ± 2 | 100 ± 2 |
| LC-WCXb | 0 | 0 | 0 | 10 ± 1 |
| LC-SCXb | 100 ± 5 | 33 ± 2 | 33 ± 2 | 98 ± 5 |
| Resin-based SCXc | 0 | 0 | 0 | 100 ± 5 |
| Silica-based SAX, QMAa | 22 ± 1 | 100 ± 5 | 100 ± 5 | 100 ± 5 |
| LC-SAXb | 10 ± 1 | 100 ± 5 | 100 ± 5 | 84 ± 4 |
| Resin based SAXc | 25 ± 1 | 90 ± 5 | 80 ± 4 | 10 ± 1 |
Cation exchange cartridges
The LC-WCX cartridge (Supelco), showed little retention of arsenic species, whereas the SCX cartridges completely retained DMA (Table 2). The silica-based, sulfonic-acid bonded, LC-SCX cartridge (Supelco) was able to retain As(III) (100%) and DMA (98%), and partially retain As(V) (33%) and MMA (33%). Among the four arsenic species, DMA is the only species that exists as a cation in acidic medium. Therefore, complete retention of DMA on SCX cartridges is reasonable. However, because of the wide range of dissociation constants, As(V) and MMA mainly exist as anionic species at almost all pH [pKa1 = 3.6, pKa2 = 8.2 for MMA and pKa1 = 2.3, pKa2 = 6.8 and pKa3 = 11.6 for As(V)]. As(III), having pKa values of larger than 9 (pKa1 = 9.3, pKa2 = 13.5 and pKa3 = 14), is not dissociated at neutral pH and is present as a neutral species, As(OH)3. Therefore, the retention of As(V), MMA, and As(III) on this SCX cartridge is surprising. The retained arsenic species were also difficult to elute from this cartridge. Thus, this SCX cartridge is not useful for selective retention and elution of arsenic species.A resin-based SCX cartridge (Alltech) was specific for DMA (100% retention), while the other species, MMA, As(V) and As(III), were unretained. This resin-based SCX cartridge is useful for separating DMA from the other three arsenic species. Subsequent elution of DMA from the resin-based SCX cartridge was quantitative by using 1.0 M HCl.
SAX cartridges
The affinity of SAX cartridges for arsenic species was tested on both silica-based (from two different suppliers) and resin-based cartridges. All the SAX cartridges showed almost complete retention of As(V) and MMA. For both types of silica-based SAX cartridges, (QMA and LC-SAX), the retention of As(V) and MMA was 100%, whereas the resin-based SAX cartridge exhibited a retention of 80–90% for As(V) and MMA.Retention of DMA varied with resin-based and silica-based SAX cartridge; with almost complete retention of DMA on both silica-based SAX cartridges (100% on QMA cartridge and 84% on LC-SAX), and only 10% retention on the resin-based SAX cartridge.
Speciation of arsenic in water
The differences in retention and elution properties of arsenic species enabled us to develop a simple speciation method. The method is based on the selective retention of arsenic species on specific cartridges followed by selective elution. Fig. 2 shows representative profiles from the speciation analysis of arsenic in bottled water that was spiked with 2 µg L−1 of As(III), As(V), MMA, or DMA. A 15 mL water sample was passed through both an SCX cartridge and an SAX cartridge in tandem. The effluent was collected for analysis of arsenic. As(III) was not retained on either cartridge and was recovered in the effluent, as demonstrated by the same signal intensity for the effluent (E1) and the 2 µg L−1 As(III) standard (S1). | ||
| Fig. 2 Comparison of arsenic signals in standard solutions, eluents and effluent. The analyses were performed using FI-HGAAS. (E1), Effluent containing unretained As(III); (S1) 2 µg L−1 As(III) standard in deionized water; (E2) eluent from SCX cartridge; (E2) 6 µg L−1 DMA standard in 1.0 M HCl; (E3) eluent from SAX cartridge with 0.1 M acetic acid; (E3) 6 µg L−1 MMA standard in 0.1 M acetic acid; (E4) eluent from SAX cartridge with 1.0 M HCl; (4b) 6 µg L−1 As(V) in 1.0 M HCl. | ||
When a 15 mL water sample containing 2 µg L−1 of DMA was passed through the SCX cartridge, no arsenic was detectable in the waste solution, indicating that DMA was completely retained on the cartridge. A 5 mL solution of 1.0 M hydrochloric acid was used to elute the DMA from the cartridge and the eluent was analyzed. As the solution volume was reduced from the initial 15 mL water to the 5 mL eluent, the concentration of DMA was expected to be 6 µg L−1 for a quantitative recovery of DMA. Indeed, the similar peak intensities for the eluent (E2) and the 6 µg L−1 DMA standard (S2) confirmed that DMA was quantitatively retained and eluted.
When a 15 mL water sample containing both As(V) and MMA (each 2 µg L−1) was passed through the LC-SAX cartridge, no arsenic was detectable in the effluent, indicating that As(V) and MMA were retained on the cartridge. Acetic acid (0.1 M, 5 mL) was used to selectively elute MMA from the cartridge. A quantitative recovery of MMA was obtained as indicated by the same signal intensity for both the eluent (E3) and the MMA standard (S3). Hydrochloric acid (1.0 M, 5 mL) was subsequently used to elute As(V) from the LC-SAX cartridge. A quantitative recovery of As(V) was obtained, as shown in Fig. 2 (E4 and S4).
The method was applied to the speciation of arsenic in water samples obtained from different stages of water treatment. As(III) and As(V) were found to be the only detectable arsenic species. MMA and DMA were not detected in these water samples. Therefore, only a single SAX cartridge was used to separate As(V) from As(III). Fig. 3 shows traces from FI-HGAFS analyses of As(V) eluted from the cartridge. The water samples were collected from seven points along the water treatment process. Triplicate aliquots (15 mL) were passed through three separate SAX cartridges on-site. Replicate analyses of the eluents from the cartridges are depicted in Fig. 3. Consistent peak intensity demonstrates the reproducibility between the cartridges and between analyses. Results for As(III) and As(V) concentration in these water samples are summarized in Table 3. Results are mean ± s for 8–10 replicate analyses of each sample.
 | ||
| Fig. 3 Representative traces from the replicate analyses of As(V) in water samples collected at seven stages of water treatment from a drinking water treatment plant. Replicate water samples were passed through SAX cartridges and the retained As(V) was eluted with 1.0 M HCl. The eluents were analyzed for arsenic using FI-HGAFS. | ||
| Sample | Treatment process | As(V) | As(III) |
|---|---|---|---|
| 1 | Raw water from river | 0.32 ± 0.01 | 0.21 ± 0.08 |
| 2 | Water from alum tank | 0.48 ± 0.01 | 0.16 ± 0.06 |
| 3 | Post-alum treatment | 0.31 ± 0.01 | 0.23 ± 0.04 |
| 4 | Post-lime treatment | 0.22 ± 0.01 | 0.28 ± 0.02 |
| 5 | Addition of fluoride; disinfection by chlorination (prefiltration) | 0.68 ± 0.04 | 0.20 ± 0.03 |
| 6 | Filtration; pH adjustment (treated water) | 0.54 ± 0.04 | 0.18 ± 0.05 |
| 7 | Tap water | 0.61 ± 0.03 | 0.17 ± 0.03 |
Speciation of arsenic in urine
The method was also applied to the speciation of urinary arsenic. Initial experiments showed that DMA, MMA, and As(V) in urine samples were not completely retained on the SCX and SAX cartridges connected in tandem. The retention of these species varied with different urine samples, suggesting matrix effects from the samples. To reduce the matrix effect, we diluted urine samples 10-fold and added a C18 cartridge before the SCX–SAX cartridges. The use of the C18 cartridge was to remove the organic matrix. The C18 cartridge did not retain any of the four target arsenic species.Using the cartridge method and the previously developed HPLC-HGAFS method26 we compared speciation of arsenic in a reference material urine (SRM 2670 Toxic Metals in Urine) and in two volunteer urine samples. Results from both methods are summarized in Table 4, and are in agreement. The urine samples were collected from a volunteer before (urine 1) and after (urine 2) the ingestion of 250 g mussels that contained arsenosugars. The increase of DMA concentration in urine after the ingestion of the mussels is due to the metabolism of the arsenosugars present in the mussels.41,42
| Samples | As(III) | As(V) | MMA | DMA | ||||
|---|---|---|---|---|---|---|---|---|
| Cartridge method | HPLC-HGAFS | Cartridge method | HPLC-HGAFS | Cartridge method | HPLC-HGAFS | Cartridge method | HPLC-HGAFS | |
| a SRM = Standard Reference Material (NIST, Gaithersburg, MD, USA).b n.d. = not detectable. | ||||||||
| SRM 2670a | n.d.b | n.d. | n.d. | n.d. | 6.5 ± 0.2 | 7.4 ± 1.2 | 40 ± 1 | 42 ± 1 |
| Urine 1 | n.d. | n.d. | n.d. | n.d. | 1.1 ± 0.1 | 1.3 ± 0.2 | 4.5 ± 0.2 | 5.1 ± 0.3 |
| Urine 2 | 2.6 ± 0.2 | 2.9 | n.d. | n.d. | 3.4 ± 0.1 | 3.9 ± 0.4 | 47 ± 1 | 50 ± 2 |
The use of tandem cartridges for the selective retention and elution of arsenic species is particularly useful for speciation of arsenic in water. Separation of arsenic species can be carried out in the field, and is suitable for routine environmental monitoring. Speciation of arsenic in urine requires a dilution of the urine sample to reduce sample matrix effects. Arsenobetaine is not detectable using the direct hydride generation process.
Acknowledgements
The authors thank Dr. L. Gamie and Ms. N. Best of EPCOR Water Services (formerly AQUALTA, Edmonton, Canada) for assistance on water sampling. They thank Dr. M. Ma of University of Alberta for providing HPLC-HGAFS analyses of the urine samples. American Water Works Association Research Foundation (USA) and Natural Sciences and Engineering Research Council of Canada supported this work.References
- National Research Council, Arsenic in Drinking Water, National Academy Press, Washington, DC, 1999. Search PubMed.
- R. S. Braman and C. C. Foreback, Science, 1973, 182, 1247 CrossRef CAS.
- M. O. Andreae, in Organometallic Compounds in the Environment, Principles and Reactions, ed. P. J. Craig, John Wiley, New York, 1986, pp. 198–228. Search PubMed.
- W. R. Cullen and K. J. Reimer, Chem. Rev., 1989, 89, 713 CrossRef.
- World Health Organization, Environmental Health Criteria 18: Arsenic, WHO, Geneva, 1981. Search PubMed.
- Agency for Toxic Substances and Disease Registry, Toxicological Profile for Arsenic, US Department of Health and Human Services, Atlanta, GA, 2000. Search PubMed.
- C.-J. Chen, Y.-C. Chuang, T.-M. Lin and H.-Y. Wu, Cancer Res., 1985, 45, 5895 Search PubMed.
- M. M. Wu, T. L. Kuo, Y. H. Hwang and C. J. Chen, J. Am. Epidemiol., 1989, 130, 1123 Search PubMed.
- C. J. Chen and J. D. Wang, Cancer Res., 1990, 50, 5470 Search PubMed.
- H. R. Guo, H. S. Chiang, H. Hu, S. R. Lipsitz and R. R. Monson, Epidemiology, 1997, 8, 545 CrossRef CAS.
- C. Hopenhayn-Rich, M. L. Biggs, A. Fuchs, R. Bergoglio, E. E. Tello, H. Nicolli and A. H. Smith, Epidemiology, 1996, 7, 117 CAS.
- C. Hopenhayn-Rich, M. L. Biggs and A. H. Smith, Int. J. Epidemiol., 1998, 27, 561 Search PubMed.
- A. H. Smith, M. Goycolea, R. Hague and M. L. Biggs, Am. J. Epidemiol., 1998, 147, 660 CAS.
- US Environmental Protection Agency, Fed. Regist., 2000, 65, 38888 Search PubMed.
- Health and Welfare Canada, Guidelines for Canadian Drinking Water Quality, Arsenic, Ministry of Health, Ottawa, 1992. Search PubMed.
- Y. Shibata, M. Morita and K. Fuwa, Adv. Biophys., 1992, 28, 31 CrossRef CAS.
- N. P. Vela, L. K. Olson and J. A. Caruso, Anal. Chem., 1993, 65, 585A CAS.
- E. H. Larsen, G. Pritzi and S. H. Hansen, J. Anal. At. Spectrom., 1993, 8, 1075 RSC.
- J. R. Dean, L. Ebdon, M. E. Foulkes, H. M. Crews and R. C. Massey, J. Anal. At. Spectrom., 1994, 9, 615 RSC.
- X. C. Le, W. R. Cullen and K. J. Reimer, Environ. Sci. Technol., 1994, 28, 1598 CAS.
- A. Geiszinger, W. Goessler, D. Kuehnelt, K. A. Francesconi and W. Kosmus, Environ. Sci. Technol., 1998, 32, 2238 CrossRef CAS.
- S. Wangkarn and S. A. Pergantis, J. Anal. At. Spectrom., 2000, 15, 627 RSC.
- A. G. Howard, J. Anal. At. Spectrom., 1997, 12, 267 RSC.
- X. Zhang, R. Cornelis, J. De Kimpe and L. Mees, Anal. Chim. Acta, 1996, 319, 177 CrossRef CAS.
- M. A. Lopez-Gonzalvez, M. M. Gomez, C. Camara and M. A. Palacios, J. Anal. At. Spectrom., 1994, 9, 291 RSC.
- X. C. Le and M. Ma, Anal. Chem., 1998, 70, 1926 CrossRef CAS.
- K. J. Lamble and S. J. Hill, Anal. Chim. Acta, 1996, 334, 261 CrossRef CAS.
- G. Rauret, R. Rubio and A. Padro, Fresenius' J. Anal. Chem., 1991, 340, 157 CrossRef CAS.
- K. W. M. Siu, G. Guevremont, J. C. Y. le Blanc, G. J. Garnder and S. S. Berman, J. Chromatogr., 1991, 554, 27 CrossRef CAS.
- J. J. Corr and E. H. Larsen, J. Anal. At. Spectrom., 1996, 11, 1215 RSC.
- S. A. Pergantis, W. Winnik and D. Betowski, J. Anal. At. Spectrom., 1997, 12, 531 RSC.
- S. A. Pergantis, S. Wangkarn, K. A. Francesconi and J. E. Thomas-Oates, Anal. Chem., 2000, 72, 357 CrossRef CAS.
- S. N. Pedersen and K. A. Francesconi, Rapid Commun. Mass Spectrom., 2000, 14, 641 CrossRef CAS.
- A. D. Madsen, W. Goessler, S. N. Pedersen and K. A. Francesconi, J. Anal. At. Spectrom., 2000, 15, 657 RSC.
- A. D. Eaton, J. Am. Water Works Assoc., 1994, 86, 100 Search PubMed.
- H.-W. Chen, M. M. Frey, D. Clifford, L. S. McNeill and M. Edwards, J. Am. Water Works Assoc., 1999, 91, 74 Search PubMed.
- S. Yalcin and X. C. Le, Talanta, 1998, 47, 787 CrossRef CAS.
- X. C. Le, M. Ma and N. A. Wong, Anal. Chem., 1996, 68, 4501 CrossRef CAS.
- M. Ma and X. C. Le, Clin. Chem., 1998, 44, 539 Search PubMed.
- J. Feldmann, V. W.-M. Lai, W. R. Cullen, M. Ma, X. Lu and X. C. Le, Clin. Chem., 1999, 45, 1988 Search PubMed.
- X. C. Le, W. R. Cullen and K. J. Reimer, Clin. Chem., 1994, 40, 617 Search PubMed.
- X. C. Le, M. Ma and V. W. M. Lai, in Arsenic Exposure and Health Effects, ed. W. R. Chappell, C. O. Abernathy and R. L. Calderon, Elsevier, Amsterdam, 1999, pp. 69–79 Search PubMed.
Footnote |
| † Presented at the Whistler 2000 Speciation Symposium, Whistler Resort, BC, Canada, June 25–July 1, 2000. |
| This journal is © The Royal Society of Chemistry 2001 |
