Sulfur speciation monitored in situ with solid state gold amalgam voltammetric microelectrodes: polysulfides as a special case in sediments, microbial mats and hydrothermal vent waters†‡
George W. Luther, III*a, Brian T. Glazera, Laura Hohmanna, Jeannette I. Poppa, Martial Taillefertb, Timothy F. Rozana, Paul J. Brendelc, Stephen M. Theberge§a and Donald B. Nuzziod
aCollege of Marine Studies, University of Delaware, Lewes,, DE 19958, USA. E-mail: luther@udel.edu
bSchool of Earth & Atmospheric Sciences, Georgia Institute of Technology, 221 Bobby Dodd Way, Atlanta,, GA 30332-0340, USA
cWard Melville High School, 380 Old Town Road, East Setauket,, NY 11733, USA
dAnalytical Instrument Systems, Inc., 1059C Old York Road, Ringoes,, NJ 08851, USA
First published on 6th December 2000
Abstract
Sulfur speciation was determined in real time in salt marsh microbial mats, subtidal sediments and hydrothermal vent diffuse flow waters using solid state gold-amalgam voltammetric microelectrodes. Chemical species were measured in situ without any sample manipulation or processing. The partially oxidized sulfur species detected were polysulfides, thiosulfate, elemental sulfur and tetrathionate. Fe(III) oxidation of hydrogen sulfide does not occur within the mats where microbially mediated processes are responsible for oxidation of H2S. In sediments and diffuse flow vent waters, Fe(III) phases are the direct oxidant of H2S. Sulfur speciation determined in this work is due to in situ biogeochemical processes and is not due to artefacts of sample manipulation. The voltammetric data show that polysulfides are the first detectable intermediate during sulfide oxidation which is consistent with previous laboratory studies.
Introduction
In situ measurements are necessary to understand dynamic environments where speciation can change in seconds as a result of chemical, biological and physical processes. A wide range of natural environments produce hydrogen sulfide that can then be oxidized back to sulfate by oxygen, iron(III) and manganese(III,IV) compounds.1–5 The oxidation may be abiotic or bacterially mediated and occur at reasonable rates (minutes to hours).1–5 The oxidation of sulfide frequently leads to intermediate oxidation state compounds, such as elemental sulfur, polysulfides and thiosulfate4,5 which may be used by bacteria6 or may react with metals7 and organic compounds.8,9 Using the mercury electrode and/or the gold amalgam microelectrode it is possible to measure a variety of soluble sulfur compounds and ions including hydrogen sulfide, polysulfides, thiosulfate, aqueous iron monosulfide, elemental sulfur, tetrathionate and organic thiols.10–16Polysulfides are key intermediates in the oxidation of H2S but could not be easily measured or even identified in situ until recently.16 Previous studies have used a dropping Hg electrode to measure polysulfides discreetly.10,17 In addition, most previous measurements of sulfur species were determined after sample manipulation. Sample manipulation includes: (i) taking a water sample in Niskin or Go-Flo bottles and measuring the speciation back in the lab (either at home or onboard ship); and (ii) taking sediment cores which are then cut into sections (typically >3 mm), centrifuged for removal, filtration and measurement of the porewater. Sample processing can mix oxidized sediments/waters and reduced sediments/waters which can react to create sulfur species of intermediate oxidation state. Although the measurements are performed well, the sample processing causes artefacts which can complicate the interpretation of what biogeochemical processes occur in the field. Thus, the exact determination of polysulfides, which appear to be the first formed or detected intermediates in sulfide oxidation,4,5 has been hampered.
In this work, we describe the electrode reactions and quantify the presence and/or absence of these species over space and time in microbial mats, sediments and hydrothermal vent fields using a solid state gold amalgam voltammetric microelectrode.18 The electrodes have (sub)millimetre vertical resolution in mats and sediments. The initial survey work that is presented here will show in situ sulfur speciation measurements where: (1) the electrodes and a portable analyzer have been brought into the field for analysis of salt marsh microbial mats; (2) subtidal sediment cores have been collected and returned to the laboratory for electrode insertion; and (3) waters at 2500 m depth have been analyzed with a submersible in situ analyzer from a deep sea submarine (DSV Alvin). Artefacts caused by sample processing are minimized and a correct assessment of the sulfur speciation including the presence of polysulfides can be made.
Experimental
A standard three electrode cell was used in all experiments. The working electrode was a gold amalgam (Au/Hg) electrode of 100 µm diameter. The electrode was made in glass or commercially available PEEK® (polyethyl ether ketone) tubing depending on the application as per Luther et al.19 Glass was used for the bacterial mat and sediment analyses because 5 mm glass could be extruded to about 0.2–0.3 mm for ease of insertion into the environmental sample.Microbial mats from Great Marsh, Delaware, during July 6, 2000, were analyzed in
situ with a gold amalgam (Au/Hg) microelectrode which was
inserted into the mat with a manually controlled three-axis Narshige micromanipulator.
The counter (Pt) and reference (Ag/AgCl in saturated KCl)
electrodes were placed in water overlying the mat so they did not enter the
sediment or sulfide zone. A battery operated DLK 100A from Analytical Instrument
Systems, Inc. was used for all electrochemical analyses. The overlying water
had a salinity of 32 at 29.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and a pH of 8.29 so that O2
saturation is 198.9 µmol dm−3. There
was no cloud cover during the experiment, which occurred between 1200 and
1400 h.
°C and a pH of 8.29 so that O2
saturation is 198.9 µmol dm−3. There
was no cloud cover during the experiment, which occurred between 1200 and
1400 h.
For subtidal sediment analyses, cores were obtained at an ambient temperature (15–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C),
returned to the laboratory and placed in a water bath/incubator for insertion
of the gold amalgam (Au/Hg) microelectrode. The reference electrode
was a standard Ag/AgCl as above and the counter-electrode was Pt wire.
The area chosen for study was northwest Rehoboth Bay of southern Delaware.
°C),
returned to the laboratory and placed in a water bath/incubator for insertion
of the gold amalgam (Au/Hg) microelectrode. The reference electrode
was a standard Ag/AgCl as above and the counter-electrode was Pt wire.
The area chosen for study was northwest Rehoboth Bay of southern Delaware.
For hydrothermal vent research at 9° N East Pacific Rise during
May 1999, a Delrin tube was made to hold up to four PEEK® electrodes
and two glass electrodes with a thermocouple and Teflon tubing to suck water
into syringes using the SIPPER sampler.20
The lower end of this tube had set screws to hold the working electrodes stationary
in the tube. The working electrodes were recessed at the lower end of the
wand to protect the tips of the electrodes from losing the Hg film. The lower
end also had feet to allow water flow across the electrode surface. The top
end of the tube was mated via four stainless steel nuts and bolts
to a large diameter tube. Inserted between these lower and upper Delrin tubes
was a steel handle for DSV Alvin's arm to pick up the entire
wand assembly for deployment of the electrodes. We refer to this as a sensor
package for simplicity. The reference electrode was Ag/AgCl and the counter-electrode
was Pt wire, both of which were mounted on the basket of DSV Alvin
so that they would not enter sulfidic waters. For hydrothermal vent work,
the Ag/AgCl reference was silver wire which was oxidized in seawater at +9 V
for 10 s to form a AgCl coating; the electrode was used as a solid
state electrode in the seawater medium (I = 0.7)
so that no pressure effects on filling solutions would hinder electrode performance.
Comparison of peak potentials for the analytes measured in situ and
onboard ship were the same and were similar to those for a saturated calomel
electrode (SCE). The sensor package was held over areas at the base
of vent chimneys where the vent tubeworm, Riftiapachyptila,
inhabits. These areas are termed diffuse flow because water temperatures are
less than 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and do not emanate from the vent orifice. A submersible
electrochemical analyzer from Analytical Instrument Systems, Inc. was used
for data collection.
°C and do not emanate from the vent orifice. A submersible
electrochemical analyzer from Analytical Instrument Systems, Inc. was used
for data collection.
Electrochemically conditioning the electrode between scans removes any chemical species from the electrode surface restoring it for use for the next measurement. To remove any previously deposited Fe and Mn; the working electrode was conditioned at a potential of −0.1 V for 10 s.18 The voltage range scanned was from −0.1 V to −2.0 V for linear sweep voltammetry (LSV) and for cyclic voltammetry (CV). In LSV or CV, the scan rate was either 200, 500 or 1000 mV s−1. In square wave voltammetry (SWV), the following set of parameters was used: pulse height, 24 mV; step increment, 1 mV; frequency, 100 Hz; scan rate, 200 mV s−1. LSV and CV were used primarily to measure O2 and sulfur species. SWV was employed for analysis of the metal redox species.
Standard calibration curves were produced in seawater over the temperature range of the samples for all sulfur species and the electrodes calibrated as per Brendel and Luther.18 Pure Na2S4 was prepared by premixing and heating elemental sulfur and anhydrous sodium sulfide in sealed and evacuated glass tubes.7 The purity of the product was confirmed by differential scanning calorimetry (DSC) analysis and the products are typically >99% pure. Individual standard solutions of sulfide and thiosulfate were made using ACS reagent grade Na2S·9H2O (Aldrich) and Na2S2O3·5H2O (Fisher). Solutions of S0 were prepared by dissolving 99.998% pure elemental sulfur (Aldrich) in HPLC grade methanol. S0 standards were then diluted to 0.001% v/v CH3OH·H2O to minimize any potential organic fouling of the Hg electrode. All laboratory prepared solutions were made with Milli-Q water that had been purged with N2 for 1 h. The N2 was first passed through a pyrocatechol trap, which lowers O2 concentrations <0.2 µmol dm−3.
Results and discussion
Sulfur species
Table 1 shows many of the redox compounds and ions including some of the sulfur species that can be measured with a Au/Hg electrode. Sulfide oxidation results in formation of polysulfides, elemental sulfur, thiosulfate, sulfite and polythionates (e.g., tetrathionate) in lab studies1–5 but the possible existence of these sulfur intermediates has only been confirmed in a few field studies.10,11,13–16 Sulfite can be measured10 at pH values <6, which are not common in microbial mats and sediments where the pH is >7. All sulfur species give one peak except for polysulfides. At slow scan speeds, H2S, S8 and polysulfides (Sx2−) overlap to give one peak at about −0.60 V and the sum of all their contributions is termed Sred.10,13–17 However, Sx2− are unique because they exist in two oxidation states and it is possible to discriminate each oxidation state with fast potential scans.16 At positive potentials, Sx2− react to form a HgSx species at the Au/Hg electrode, which is an electrochemical oxidation of the Hg. On scanning negatively, HgSx is reduced to Hg and Sx2− at a more positive potential that overlaps with H2S and S8, then the (x−1)S0 atoms are reduced to sulfide at a more negative potential. Because the reduction of S0 atoms in Sx2− is an irreversible process, increasing the scan rate shifts that peak to more negative potentials and permits separation of the HgSx reduction from the S8 reduction [Fig. 1; Table 1, eqns. (4a)–(4c)].16,21 The peaks can be fully resolved at scan rates of 1000 mV s−1. On the positive scan in the polysulfide CV, all the S0 that was reduced to sulfide and the S2− in the polysulfide react to reform a HgS film (termed SAVS). Lastly, an irreversible peak for the reduction of Fe2+ in soluble FeS, FeSaq, can be observed at −1.10 V.12 | ||
| Fig. 1 Separation of the tetrasulfide signal into S0 and S2− signals as scan rate is increased. At the 1000 mV s−1 scan rate, two signals are readily observable with a ratio of 3 ∶ 1 for S0 to S2− with an increase in signal intensity as expected. These data were obtained with a hanging Hg drop electrode. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C a
°C a
| Ep/V | MDL/µmol dm−3 | ||
|---|---|---|---|
| a Potentials versus the Ag/AgCl are 44 mV more positive. All data were obtained with a 100 µm diameter electrode (A = 7.85 × 10−3 mm2). O2 data were collected by LSV at 200 mV s−1; all others were collected by SWV at 200 mV s−1. Potentials can vary with scan rate and concentration; e.g., on increasing concentration, the sulfide signal becomes more negative.7,16 When applying potential from a positive to negative scan direction, sulfide and S0 react in a two step process (1, adsorption onto the Hg surface; 2, reduction of the HgS film) and polysulfides react in a three step process (1, adsorption onto the Hg surface; 2, reduction of the HgSx film; 3, reduction of the S0 in the polysulfide). Increasing the scan rate separates electrode reactions (4b) and (4c) into two peaks because eqn. (4c) is an irreversible process (increasing scan rate shifts this signal21). MDL = minimum detection limit. | |||
| (1a) | O2 + 2H+ + 2e− → H2O2 | −0.30 | 5 |
| (1b) | H2O2 + 2H+ + 2e− → H2O | −1.3 | 5 |
| (2a) | HS− + Hg → HgS + H+ + 2e− | Adsorption onto Hg < −0.60 | |
| (2b) | HgS + H+ + 2e− ↔ HS− + Hg | ∼−0.60 | <0.2 |
| (3a) | S0 + Hg → HgS | Adsorption onto Hg < −0.60 | |
| (3b) | HgS + H+ + 2e− ↔ HS− + Hg | ∼−0.60 | <0.2 |
| (4a) | Hg + Sx2− → HgSx + 2e− | Adsorption onto Hg < −0.60 | |
| (4b) | HgSx + 2e− ↔ Hg + Sx2− | ∼−0.60 | <0.2 |
| (4c) | Sx2− + xH+ + (2x − 2)e− → xHS− | ∼−0.60 | <0.2 |
| (5) | 2RSH ↔ Hg(SR)2 + 2H+ + 2e− | Typically more positive than H2S/HS− | |
| (6) | 2S2O32− + Hg ↔ Hg(S2O3)22− + 2e− | −0.15 | 16 |
| (7) | S4O62− + 2e− → 2S2O32− | −0.45 | 15 |
| (8) | FeS + 2e− + H+ → Fe(Hg) + HS− | −1.1 | Molecular species |
| (9) | Fe2+ + Hg + 2e− ↔ Fe(Hg) | −1.43 | 15 |
| (10) | Fe3+ + e− ↔ Fe2+ | −0.2 to −0.9 V | Molecular species |
In situ mat work
Fig. 2 shows representative voltammograms taken from a microbial mat in Great Marsh Delaware. Scan A clearly shows the existence of only oxygen above the mat (4 mm) whereas scan B shows a significant signal due to H2O2 at 0–2 mm above the mat. The H2O2 signal is normally equal to the O2 signal because, at the Hg electrode, O2 reduces to H2O2 [Table 1, eqns. (1a)–(1b)] which in turn reduces to H2O. Peroxide has been shown to exist in significant quantities in biofilms.22 Scan C shows two peaks: one for thiosulfate, S2O32− (E½ = −0.15 V), and Sred (E½ = −0.60 V) at 2 mm below the mat surface. Fast scans of 1000 mV s−1 only show one peak indicating that polysulfides are not present as part of Sred. Thus, Sred in this case is composed of soluble S8 because this upper part of the mat is more oxidized due to the oxygen gradient. Deeper into the mat (5 mm), scan D, shows a clear double peak at E½ = −0.58 V and −0.68 V indicative of polysulfide formation. The more positive signal is due to S2− sulfur from H2S and Sx2− and the more negative signal is due to S0 sulfur from Sx2−. Polysulfides persist in the bottom portion of the mat where purple sulfur bacteria exist. As the bottom of the mat is penetrated and the sediment is reached, only one peak at E½ = −0.58 V is observed in scan E (1.5 mm below the mat/sediment interface), which indicates only H2S/HS− is present. Traces of FeSaq are noted in scan E and other scans deeper into the sediment (data not shown). In none of these scans is there evidence for dissolved Fe and Mn in the mat. | ||
| Fig. 2 Representative LSV and CV scans showing sulfur speciation from a salt marsh microbial mat. A, LSV of 500 mV s−1 scan rate showing O2 in overlying water; B, LSV of 500 mV s−1 scan rate showing H2O2 at the mat/water interface; C, CV of 1000 mV s−1 scan rate showing S8 and S2O32− as the electrode penetrates through the green algae section; D, CV of 1000 mV s−1 scan rate showing Sx2− signals from S0 and S2−; E, CV of 1000 mV s−1 scan rate showing only H2S and a trace of FeSaq as the electrode penetrates the sediment. | ||
Fig. 3 shows a profile of oxygen and sulfur species into the mat which is about 7 mm thick. The O2 profile is typical for microbial mats.23–26 O2 in the water column reaches a maximum above the mat/water interface as in previous studies. During the day, O2 increases due to oxygenic photosynthesis and creates the levels of (super)saturation (∼200%) shown in Fig. 3. These levels drive the much larger O2 fluxes from the surface of the mat into the water column, thus forming a maximum at or slightly above the mat/water interface. However, as the mat/water interface was reached, H2O2 an intermediate in O2 formation via water splitting was observed. Previous microelectrode studies using membrane electrodes rather than solid state electrodes could not determine if H2O2 was present, but solid state microelectrodes used in biofilm work22 have shown the presence of peroxide.
 | ||
| Fig. 3 Profile of all chemical components measured in the microbial mat. When a chemical species is no longer detected, it is not plotted. Sred is the peak at −0.58 V and is the sum of all sulfur in the 2− oxidation state from polysulfides and H2S/HS− and in the 0 oxidation state from S8. Note that the data points for these are connected in the figure to show the transition from S8 to Sx2− to H2S. | ||
These data clearly show that there is a redox transition between the overlying water, the mat and the sediment as expected. The transition from reduced sulfide below the mat to partially oxidized sulfur species (Sx2−) in the center of the mat to more oxidized sulfur species towards the top of the mat can be explained by the biology and chemistry of the mat. Table 2 shows a schematic representation of the chemistry of a microbial mat. Visual inspection indicated that green algae/cyanobacteria (6 mm thick), which produce O2 and organic matter during photosynthesis, inhabited the top of the mat (7 mm total thickness). Underneath the algae were purple sulfur bacteria (PSB; 1 mm thick) which use H2S to produce S8 (S0) and organic matter. The organic matter from the cyanobacteria and purple sulfur bacteria is the fuel for dissimilatory sulfate reducing bacteria which lie in the sediments underneath the PSB. Although the sediments contain significant quantities of solid phase Fe(II,III) which react with sulfide to form FeS and FeS2, we did not observe a significant signal for FeSaq in this mat. FeSaq is found readily in the black colored sediments.
| Process | Reaction | Pathway | |
|---|---|---|---|
| Oxygenic photosynthesis | CO2 + H2O → CH2O + O2 | Algae/cyanobacteria | |
| Anoxygenic photosynthesis | CO2 + 2H2S → CH2O + H2O + 2S0 | Purple sulfur bacteria | |
| Sulfate reduction | 2(CH2O) + SO42− → 2HCO3− + H2S | Sulfate reducing bacteria | |
| Fe chemistry | 2Fe3+ + H2S → 2Fe2+ + 2H+ + S0 (Sx2−) | Chemical reaction | |
| Fe2+ + H2S → FeS + 2H+ | |||
| Fe2+ + Sx2− → FeS + (x − 1)S8 | |||
| FeS + H2S → FeS2 + H2 | |||
| Sulfur oxidation | 2H2S + µO2 + Fe2+ → | S2O32−; S8; Sx2−; SO32−; SO42−; S4O62− | Chemical reaction and/or sulfur oxidizing bacteria |
| H2S + Fe3+→ | |||
The mat surface produces oxidants, O2 and H2O2, which are able to oxidize sulfur compounds whereas the PSB mediate sulfide oxidation directly. The formation of S8 and S2O32− at the upper portions of the mat is consistent with the stronger oxidizing characteristics of that section of the mat where oxygenic photosynthesis occurs. Polysulfides form in the interior of the mat and their formation likely occurs from the reaction of HS− with S8 that PSB produce during anoxygenic organic matter production. The formation of these partially oxidized sulfur species is the result of the oxidation of H2S/HS− which diffuses from the reducing sediments toward the overlying water. The data set is consistent with laboratory studies of sulfide oxidation5 that show formation of polysulfides early in the oxidation and production of thiosulfate later. In Fig. 3, polysulfides but not thiosulfate are observed deep in the mat whereas thiosulfate but not polysulfides are observed nearer the mat surface.
While colorimetric analyses25,26 have been employed to measure sulfur species such as thiosulfate, tetrathionate and polysulfides in lab cultures and processed microbial mat cores on thick films that could be cut and analyzed, this is the first report of in situ sulfur species other than H2S in a microbial mat and their occurrence occurs where predicted. The oxidation appears primarily due to biological processes because no metals (Fe, Mn), which could oxidize sulfide, were detected and O2 did not co-exist with H2S.
Subtidal sediment work
Similar sulfur speciation was also found in subtidal sediments (Fig. 4) from Rehoboth Bay, Delaware. In these submersed sediments, there are no bacterial mats present and O2 penetrates to only about 2 mm throughout the year. Sulfate reduction does occur and the resulting sulfide reacts with Fe(III) and Mn(IV) phases, which range in concentration from 10 to100 µmol (g dry wt)−1, to produce partially oxidized sulfur species since O2 does not penetrate deeply. During Spring, similar speciation was found in these sediments as in the mats except that the sulfur species were found very deep into the core where Fe chemistry dominates. An upper zone consisted primarily of S8, and dissolved Fe phases including Fe2+, FeSaq and Fe(III)–organic complexes. Soluble Fe(III) phases have been found in previous porewater studies.27 A middle zone consisted of Sx2− and FeSaq, and finally, a lower completely reduced zone composed of H2S/HS− and FeSaq. The middle zone of polysulfides was only about 5 mm over the 8 cm depth range measured as compared to 4 mm over a 2 cm microbial mat studied. Interestingly a large peak near −0.95 V was also observed in the sediments. We have only been able to reproduce this signal in laboratory solutions of polysulfides that are allowed to oxidize slowly in air. As the oxidation proceeds, white and yellow forms of elemental sulfur are visible in the solution. We hypothesize that the excess elemental sulfur adsorbs to the Hg electrode and interacts with the polysulfide in a donor–acceptor complex (Sx2− → S8 or HS− → S8) that gives rise to the signal. | ||
| Fig. 4 Representative CV scans showing sulfur speciation from a subtidal sediment. A, 1000 mV s−1 scan rate of the upper zone showing Fe(III), S8, FeSaq; B, 1000 mV s−1 scan rate of the middle area showing the Sx2− signals from S0 and S2−; C, 1000 mV s−1 scan rate showing only H2S and FeSaq as the major components. | ||
Although Fe(III) and Mn(IV) solid phases are present in abundance [total of ∼100 µmol (g dry wt)−1] in these sediments, solid phases are less reactive than soluble compounds.3,27 Thus, the combination of slower solid phase reactivity and less microbial oxidation relative to microbial mats provides less dramatic sulfur speciation in these subtidal sediments than in the salt marsh mats. The measurement of dissolved Fe compounds in these subtidal porewaters is in contrast to the salt marsh mat work described above and clearly shows that Fe chemistry is not an important component of mat biogeochemistry. Fe2+ also reacts quickly with polysulfides and at high micromolar (µmol dm−3) concentrations reacts to form FeS and S8.7 Thus, polysulfides should not reach high levels when dissolved metals are present.
In situ hydrothermal vent work
In order to assess sulfur speciation at vents, we placed the sensor package near the plumes of the vent tubeworms Riftiapachyptila, which reside in diffuse flow waters at the base or on the lower walls of the vents. These tubeworms grow up to 2 m tall and harbor bacterial endosymbionts. The primary source of organic carbon for their growth is from the bacterially mediated chemosynthetic reaction of H2S/HS− with CO2 by the endosymbionts, and the R. pachyptila move their plumes into diffuse flow waters that contain H2S/HS−. However, the formation of S8 from chemosynthesis occurs within the bacteria residing in R. pachyptila not outside Riftia. For partially oxidized sulfur species to form in the vent waters around R. pachyptila, oxygen or Fe(III) formed from Fe(II) oxidation by oxygen must react with H2S, both H2S and Fe(II) diffuse from the vent area.Figs. 5A and B show CV data and Fig. 5C time course data over a 2.5 min
period at 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Fig. 6
shows the sensor package in one of the manipulators of DSV Alvin
above R. pachyptila. The first scans showed that O2 and
H2S co-exist whereas later on in the in situ experiment,
the sulfide peak split into two peaks indicating that polysulfides formed.
S0 from polysulfides typically are about 25% or less of
the total S found near the plumes so that the S2− sulfur
due to H2S/HS− is about 70% of the
total sulfur measured. The sulfur speciation changed in a matter of seconds
and indicates that O2 is not the direct oxidant of H2S
under diffuse flow conditions. Fe is in trace quantities (∼1 µmol dm−3)
in these diffuse flow waters as determined by analysis of discrete samples
onboard ship using the Fe–ferrozine assay.28
The pH of these waters is typically between 6 and 6.8 where Fe2+
oxidation by O2 is faster than H2S oxidation by O2.29,30 Moreover, Fe(III)
formed from the reaction of Fe(II) with O2
is the likely oxidant because the transfer of a single electron from H2S
to O2 is thermodynamically unfavorable31
and Fe2+ catalysis of sulfide oxidation in oxygenated waters
is very fast.32 The Fe(III),
which formed in real time, is likely a soluble form that is very reactive27 and can produce polysulfides in a matter of seconds.
Thiosulfate, if present, was below the detection limit in these waters.
°C. Fig. 6
shows the sensor package in one of the manipulators of DSV Alvin
above R. pachyptila. The first scans showed that O2 and
H2S co-exist whereas later on in the in situ experiment,
the sulfide peak split into two peaks indicating that polysulfides formed.
S0 from polysulfides typically are about 25% or less of
the total S found near the plumes so that the S2− sulfur
due to H2S/HS− is about 70% of the
total sulfur measured. The sulfur speciation changed in a matter of seconds
and indicates that O2 is not the direct oxidant of H2S
under diffuse flow conditions. Fe is in trace quantities (∼1 µmol dm−3)
in these diffuse flow waters as determined by analysis of discrete samples
onboard ship using the Fe–ferrozine assay.28
The pH of these waters is typically between 6 and 6.8 where Fe2+
oxidation by O2 is faster than H2S oxidation by O2.29,30 Moreover, Fe(III)
formed from the reaction of Fe(II) with O2
is the likely oxidant because the transfer of a single electron from H2S
to O2 is thermodynamically unfavorable31
and Fe2+ catalysis of sulfide oxidation in oxygenated waters
is very fast.32 The Fe(III),
which formed in real time, is likely a soluble form that is very reactive27 and can produce polysulfides in a matter of seconds.
Thiosulfate, if present, was below the detection limit in these waters.
 | ||
| Fig. 5 Representative CV scans showing sulfur speciation from diffuse flow area near the vent tubeworms R. pachyptila. A, Initial 1000 mV s−1 scans showing that only O2 and H2S co-exist; B, subsequent 1000 mV s−1 scans showing that O2, H2S and Sx2− signals from S0 and S2− are observable; C, time course showing the rapid change in sulfur speciation at the tubeworm location. | ||
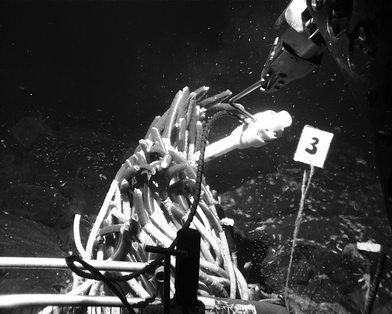 | ||
| Fig. 6 Deployment of the sensor package above R. pachyptila. (For a colour version of this figure see Electronic Supplementary Information.‡) | ||
Conclusions
The sulfur compounds of intermediate oxidation state (which are typically found in microbial mats, subtidal sediments, and hydrothermal vent waters above the electrode’s detection limits) are thiosulfate, polysulfides and aqueous elemental sulfur. Trace amounts of tetrathionate (data not shown) have also been detected. These partially oxidized sulfur species are found at redox transition zones where both an oxidant (e.g., O2) and a reductant (e.g., H2S) can be found or where biological mediation can occur. Fe(III) phases are important in the oxidation of H2S and can form quickly as dissolved components when O2 and Fe2+ react in waters of pH > 6. The major reduced dissolved sulfur species found are hydrogen sulfide and/or aqueous iron monosulfide; thiols were not detected in these study areas. In microbial mats, our data confirms that Fe and Mn chemistry are not significant in the oxidation of H2S. Because these measurements were made in real time and in the field without sample processing and/or manipulation, sulfur speciation is caused by in situ biogeochemical processes and is not due to artefacts. Polysulfides are the first detected sulfur species during H2S oxidation in these field studies and can form in seconds to minutes at circumneutral pH when dissolved Fe and O2 coexist as in hydrothermal vent diffuse flow waters.Acknowledgements
This work was supported by grants from the National Oceanic and Atmospheric Administration (NA16RG0162-03), the National Science Foundation (OCE-9714302) and a NSF Postdoctoral award to T.F. Rozan. We would like to thank David Rickard and Anthony Oldroyd for providing us with pure sodium tetrasulfide, and S. Craig Cary for contributions to our hydrothermal vent work.References
- W. Yao and F. J. Millero, Geochim. Cosmochim. Acta, 1993, 57, 3359 CrossRef CAS.
- W. Yao and F. J. Millero, Mar. Chem., 1996, 52, 1 CrossRef CAS.
- M. dos Santos Afonso and W. Stumm, Langmuir, 1992, 8, 1671 CrossRef CAS.
- M. R. Hoffmann, Environ. Sci. Technol., 1977, 11, 61 CAS.
- K. Y. Chen and J. C. Morris, Environ. Sci. Technol., 1972, 6, 529 CAS.
- B. B. Jorgensen, Science, 1990, 249, 152.
- S. J. Chadwell, D. Rickard and G. W. Luther, III, Aquat. Geochem., 1999, 5, 29 Search PubMed.
- A. Vairavamurthy and K. Mopper, in Biogenic Sulfur in the Environment, ed. E. Saltzmann and W. J. Copper, ACS, Washington, DC, 1989, vol. 393, ch. 15, pp. 231–242. Search PubMed.
- R. T. Lalonde, L. M. Ferrara and M. P. Hayes, Org. Geochem, 1987, 11, 563 CrossRef CAS.
- G. W. Luther, III, A. E. Giblin and R. Varsolona, Limnol. Oceanogr., 1985, 30, 727 Search PubMed.
- G. W. Luther, III, T. E. Church, A. E. Giblin, and R. W. Howarth, in Organic Marine Geochemistry, ed. M. Sohn, ACS, Washington, DC, 1986, vol. 305, ch. 20, pp. 340–355. Search PubMed.
- S. M. Theberge and G. W. Luther, III, Aquat. Geochem., 1997, 3, 191 Search PubMed.
- N. Batina, I. Cigenecki and B. Cosovic, Anal. Chim. Acta, 1992, 267, 157 CrossRef CAS.
- I. Ciglenečki and B. Ćosović, Electroanalysis, 1997, 9, 775 CAS.
- F. A. Wang, Tessier and J. Buffle, Limnol. Oceanogr., 1998, 43, 1353 Search PubMed.
- T. F. Rozan, S. M. Theberge and G. W. Luther, III, Anal. Chim. Acta, 2000, 415, 175 CrossRef CAS.
- J. Jordan, J. Talbott and J. Yakupkovic, Anal. Lett., 1989, 22, 1537 CAS.
- P. J. Brendel and G. W. Luther, III, Environ. Sci. Technol., 1995, 29, 751 CAS.
- G. W. Luther, III, C. E. Reimers, D. B. Nuzzio and D. Lovalvo, Environ. Sci. Technol., 1999, 33, 4352 CrossRef.
- C. A. Di Meo, J. R. Wakefield and S. C. Cary, Deep-Sea Res., Part I, 1999, 46, 1279 Search PubMed.
- A. M. Bond, Modern Polarographic Methods in Analytical Chemistry, Marcel Dekker, New York, 1980. Search PubMed.
- K. Xu, S. C. Dexter and G. W. Luther, III, Corrosion, 1998, 54, 814 Search PubMed.
- H. van Gemerden, Mar. Geol., 1993, 113, 3 CrossRef CAS.
- N. P. Revsbech and B. B. Jørgensen, Limnol. Oceanogr., 1983, 28, 749 Search PubMed.
- F. van den Ende and H. Van Gemerden, FEMS Microbiol. Ecol., 1993, 13, 69 CrossRef CAS.
- P. T. Visscher, J. W. Nijurg and H. Van Gemerden, Arch. Microbiol., 1990, 155, 75 CrossRef CAS.
- M. Taillefert, A. B. Bono and G. W. Luther, III, Environ. Sci. Technol., 2000, 34, 2169 CrossRef CAS.
- L. L. Stookey, Anal. Chem., 1970, 41, 779 CrossRef.
- F. J. Millero, S. Hubinger, M. Fernandez and S. Garnett, Environ. Sci. Technol., 1987, 21, 439 CAS.
- F. J. Millero, S. Sotolongo and M. Izaguirre, Geochim. Cosmochim. Acta, 1987, 51, 793 CrossRef CAS.
- G. W. Luther, III and T. G. Ferdelman, Environ. Sci. Technol., 1993, 27, 1154.
- F. G. Vazquez, J. Zhang and F. J. Millero, Geophys. Res. Lett., 1989, 16, 1363.
Footnotes |
| † Presented at the Whistler 2000 Speciation Symposium, Whistler Resort, BC, Canada, June 25–July 1, 2000. |
| ‡ Electronic Supplementary Information available. See http: //www.rsc.org/suppdata/em/b0/b006499h/ |
| § Present address: Dept. of Chemistry, Merrimack College, 315 Turnpike Street, N. Andover, MA 01845, USA. |
| This journal is © The Royal Society of Chemistry 2001 |
